Journal of Hepatology
Volume 56, Issue 2 , Pages 455-463, February 2012
Patrice Cacoub, Marc Bourlière, Jann Lübbe, Nicolas Dupin, Peter Buggisch, Geoffrey Dusheiko, Christophe Hézode, Odile Picard, Ramon Pujol, Siegfried Segaert, Bing Thio, Jean-Claude Roujeau
Received 23 June 2011; received in revised form 26 July 2011; accepted 2 August 2011. published online 30 August 2011.
Summary
Dermatological adverse events (AEs) are an existing concern during hepatitis C virus (HCV) infection and peginterferon/ribavirin treatment. HCV infection leads to dermatological and muco-cutaneous manifestations including small-vessel vasculitis as part of the mixed cryoglobulinemic syndrome. Peginterferon/ribavirin treatment is associated with well-characterized dermatological AEs tending towards a uniform entity of dermatitis. New direct-acting antivirals have led to significant improvements in sustained virologic response rates, but several have led to an increase in dermatological AEs versus peginterferon/ribavirin alone. In telaprevir trials, approximately half of treated patients had rash. More than 90% of these events were Grade 1 or 2 (mild/moderate) and in the majority (92%) of cases, progression to a more severe grade did not occur. In a small number of cases (6%), rash led to telaprevir discontinuation, whereupon symptoms commonly resolved. Dermatological AEs with telaprevir-based triple therapy were generally similar to those observed with peginterferon/ribavirin (xerosis, pruritus, and eczema). A few cases were classified as severe cutaneous adverse reaction (SCAR), also referred to as serious skin reactions, a group of rare conditions that are potentially life-threatening. It is therefore important to distinguish between telaprevir-related dermatitis and SCAR. The telaprevir prescribing information does not require telaprevir discontinuation for Grade 1 or 2 (mild/moderate) rash, which can be treated using emollients/moisturizers and topical corticosteroids. For Grade 3 rash, the prescribing information mandates immediate telaprevir discontinuation, with ribavirin interruption (with or without peginterferon) within 7 days of stopping telaprevir if there is no improvement, or sooner if it worsens. In case of suspicion or confirmed diagnosis of SCAR, all study medication must be discontinued.
days of stopping telaprevir if there is no improvement, or sooner if it worsens. In case of suspicion or confirmed diagnosis of SCAR, all study medication must be discontinued.
Introduction
Infection with the hepatitis C virus (HCV) results in various clinical manifestations in addition to inflammatory and fibrotic injury to the liver [1], [2]. Common among these are dermatological conditions and systemic disorders affecting the skin [3]. In some cases, cutaneous signs or symptoms may provide the first and only clue to the existence of an underlying HCV infection [4]. Treatment of dermatological manifestations of HCV through eradication of the virus is therefore important in effective patient management, although this alone is not a major justification for HCV treatment [5]. Existing and in-development antiviral therapies, however, are also associated with dermatological adverse events (AEs). In addition to reviewing the dermatological manifestations of HCV and its treatments, this paper provides practical guidance on the diagnosis and appropriate management of rash events during treatment with the recently approved HCV protease inhibitor telaprevir, in order that their impact on treatment outcomes can be limited.
Cutaneous diseases strongly linked to HCV infection
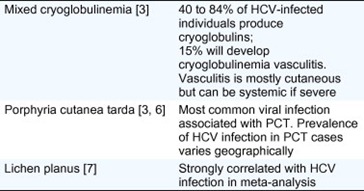
There are several cutaneous conditions that have a strong association with HCV infection. These are outlined below and summarized in Table 1.
Table 1. Cutaneous diseases strongly linked to HCV infection.
Mixed cryoglobulinemia
Mixed cryoglobulinemia (MC) is a systemic vasculitis that affects mainly the small and, less frequently, medium-sized vessels and is attributable to the expansion of B cells producing pathogenic immunoglobulin M (IgM) with rheumatoid factor (RF) activity. MC leads to clinical manifestations ranging from the so-called MC syndrome (purpura often with skin ulcers, arthralgia, and asthenia) to lesions with neurological and renal involvement due to small-vessel vasculitis [8], [9]. In a prospective study of 1614 HCV-infected patients, 40% experienced MC, and 15% developed MC vasculitis [3] Up to 80–90% of MC vasculitis cases are associated with HCV infection [8], [9] In addition to eradication of HCV infection and symptomatic alleviation, treatment of MC aims to suppress B-cell clonal expansion and cryoglobulin production. The choice of the most appropriate treatment is dependent on the extent of disease activity and organ involvement [8], [10].
Porphyria cutanea tarda
HCV is the most common viral infection associated with porphyria cutanea tarda (PCT), reported in 70–90% of PCT cases in southern Europe and 20% in northern Europe where infection is less prevalent and sunlight exposure is lower [6]. Presentation usually involves vesiculobullous eruption on skin exposed to ultraviolet light such as the back of the hands and the face, caused by deposits of uro- and heptacarboxy-porphyrins in the skin, which promote photon-driven formation of singlet oxygen species [6], [11]. These excess porphyrins are produced chiefly in the liver, and impaired liver function relating to high hepatic iron levels may provide a clue to a causal link between HCV and PCT that is yet to be fully established. Ribavirin-associated haemolysis will increase the iron load in treated patients with chronic hepatitis C and may trigger symptomatic PCT.
Lichen planus
Like PCT, the causal relationship between lichen planus and HCV is unclear [12] Nevertheless, a recent Cochrane meta-analysis found strong correlation between the two conditions. The risk of HCV infection was significantly higher for patients with lichen planus than for those without, while individuals infected with HCV also had an increased risk of having lichen planus [7].
Pruritus and other skin conditions
While pruritus is reported frequently in HCV-infected individuals, [3], [13], [14], [15] it is also a symptom of a range of hepatic co-morbitities that are common in HCV-infected individuals. It is not possible, therefore, to rule out other liver-related causes for pruritus besides the HCV infection itself [6], [16].
Association with HCV infection has been suggested for cutaneous polyarteris nodosa [17], and for a variety of other dermatological conditions including psoriasis, urticaria, and erythema multiforme (EM) [6]. However, most reported associations lacked sufficient evidence to establish a strong causal link with HCV.
Dermatological adverse events on peginterferon/ribavirin-based HCV treatment
Dermatological AEs with pegylated interferon alfa-2a or alfa-2b plus ribavirin are well known, accounting for >10% of all interferon-associated side effects [18]. There is some overlap between the safety profile of interferon-based regimens and other HCV-associated dermatological conditions, meaning distinguishing between infection and treatment in terms of causality may be difficult [16]. Other miscellaneous side effects have been reported, such as hair growth abnormalities and skin pigmentation, and are reviewed elsewhere [16].
Interferon monotherapy has dermatological side effects [19], which can be classified into localized (limited to the injection site) and generalized reactions (Table 2) [16]. Addition of ribavirin to the interferon therapy further increases the risk of dermatitis compared with interferon monotherapy (risk ratio (RR) 1.67, 95% confidence interval (CI) 1.21–2.30), including pruritus (RR 1.62, 95% CI 1.29–2.02), and rash (RR 1.74, 95%CI 1.17–2.61), as demonstrated in a recent Cochrane meta-analysis [25].
Table 2. Localized and generalized cutaneous reactions to interferon. [16], [19], [20], [21], [16], [22], [19], [23], [24]

Dermatological adverse events with peginterferon/ribavirin combination therapy tend towards a uniform entity of dermatitis, characterized by generalized pruritus and skin xerosis, with eczematiform lesions accentuated by erythematous papules and microvesicles that are often excoriated, predominantly located on the extremities and on truncal skin sites exposed to friction [22]. Management of these eruptions can be achieved with the same approach as for eczema (topical corticosteroids and emollients), usually without the need for discontinuation of the antiviral treatment [16].
Skin reactions with HCV direct-acting antiviral agents
The recent approval by the US FDA of the new HCV direct-acting antivirals (DAAs) Boceprevir [26], and Telaprevir [27] as part of triple combination therapy with the existing peginterferon/ribavirin regimen has begun a new era in HCV treatment. Phase III trials of DAA-based combination therapy in treatment-naïve and previously treated HCV genotype 1-infected patients indicate that significant improvements in sustained virological response rates can be achieved compared with peginterferon/ribavirin alone [28], [29], [30], [31]. Furthermore, DAAs offer the potential to reduce overall treatment duration to less than 48 weeks in around half of treatment-naïve patients.
weeks in around half of treatment-naïve patients.
The new treatment era, however, will bring additional patient management considerations for HCV-treating physicians. Dermatological AEs in particular have been reported with a higher frequency in trials of the HCV protease inhibitors telaprevir, [28], [29], [32], [33], [34] boceprevir [30], [31], and BI 201335 [35] as part of triple combination regimens than with peginterferon/ribavirin alone. Furthermore, rash and photosensitivity with BI 201335 appeared to be dose-dependent in Phase IIb trials, with higher rates of moderate and severe rash, and discontinuation due to rash and photosensitivity, reported in patients receiving a higher dose [35]. The mechanism of these side effects is currently unclear, although these preliminary data suggest that the management of dermatological reactions will remain important going forwards.
Data from Phase II/III telaprevir clinical trials
Primary efficacy and safety results from five placebo-controlled Phase II/III trials of telaprevir (PROVE1, PROVE2, PROVE3, ADVANCE, and REALIZE), in which 2012 patients received at least one dose of telaprevir and 764 patients received at least one dose of placebo, have recently been reported in detail [28], [29], [32], [33], [34]. Within this population, 1346 patients received the standard dose of telaprevir: 750 mg every 8
mg every 8 h, for 12
h, for 12 weeks, in combination with peginterferon/ribavirin, followed by peginterferon/ribavirin alone (T12PR). Herein we describe a pooled analysis of the dermatological safety profile of telaprevir in these patients.
weeks, in combination with peginterferon/ribavirin, followed by peginterferon/ribavirin alone (T12PR). Herein we describe a pooled analysis of the dermatological safety profile of telaprevir in these patients.
Dermatological AEs were recorded using special search categories (SSC) for ‘rash’ and ‘pruritus’. A full characterization of the skin eruptions, and potential underlying mechanisms, will be presented elsewhere, but the majority of events recorded with the ‘rash’ SSC term can be more accurately described as eczematous dermatitis, associated with pruritus, and xerosis. Here, however, we use the SSC terms ‘rash’ and pruritus consistent with the reporting of the clinical trial results.
During the telaprevir/placebo treatment phase, rash, and pruritus were among the AEs occurring more frequently (>5% difference) with telaprevir than placebo. During the telaprevir/placebo dosing phase, 55% and 51% of patients treated with T12PR had rash and pruritus, respectively, compared with 33% and 26% of placebo-treated patients (Fig. 1A).

Fig. 1. Incidence of rash (SSC) in telaprevir Phase II/III placebo-controlled trials in patients receiving telaprevir for 12 weeks in combination with peginterferon/ribavirin, followed by peginterferon/ribavirin alone (T12PR). (A) Overall incidence during the telaprevir/placebo treatment phase in the T12PR and PR arms; (B) incidence by Grade during the telaprevir treatment phase in the T12PR arms only; (C) incidence of rash in the T12PR and PR arms during the telaprevir/placebo treatment phase by 4-week periods and during the overall treatment phase by 12-week periods [36], [37].
weeks in combination with peginterferon/ribavirin, followed by peginterferon/ribavirin alone (T12PR). (A) Overall incidence during the telaprevir/placebo treatment phase in the T12PR and PR arms; (B) incidence by Grade during the telaprevir treatment phase in the T12PR arms only; (C) incidence of rash in the T12PR and PR arms during the telaprevir/placebo treatment phase by 4-week periods and during the overall treatment phase by 12-week periods [36], [37].
In the telaprevir trials, rash events were graded by severity into four grades (Table 3). More than 90% of rash (SSC) events with telaprevir were Grade 1 or 2 (mild/moderate). Of the 746 (55%) cases of rash (SSC), 495, 186, and 65 were Grades 1, 2, and 3, respectively, representing 37%, 14%, and 5% of the overall T12PR-treated population (Fig. 1B). Examples of Grades 1 and 2 dermatitis are shown in Fig. 2. In the majority (92%) of cases, progression of rash to a more severe grade did not occur [36]. A small proportion (6% [78/1346]) of all T12PR-treated patients required discontinuation of telaprevir as a result of skin conditions. Following treatment discontinuation, symptoms commonly resolved.
Table 3. Grading of telaprevir-associated rash severity in Phase III telaprevir trials [28], [29]. (Click to enlarge)
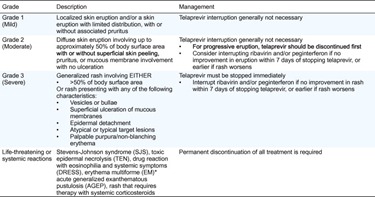
* EM is not life threatening. Careful consideration of discontinuing treatment is needed if the reaction appears different to the general dermatitis/rash, gives rise to suspicion of SJS/TEN or DRESS, or progresses in severity.

Fig. 2. Examples of (A) Grade 1 dermatitis, (B) Grade 2 dermatitis and (C) DRESS reactions to telaprevir-based therapy.
The incidence of rash (SSC) during the telaprevir/placebo phase and the overall treatment phase are shown in Fig. 1C [37]. Approximately 50% of rash events started during the first 4 weeks, with the remaining 50% starting between weeks 5–12. The median time to onset of rash (any grade) was 25 (range 1–350) days [36]. Therefore, skin eruptions can occur at any time during telaprevir treatment. Following the end of telaprevir dosing at week 12, all patients continued to receive peginterferon/ribavirin, whereupon it is noticeable that the incidence of rash was comparable between telaprevir and placebo-treated patients.
weeks, with the remaining 50% starting between weeks 5–12. The median time to onset of rash (any grade) was 25 (range 1–350) days [36]. Therefore, skin eruptions can occur at any time during telaprevir treatment. Following the end of telaprevir dosing at week 12, all patients continued to receive peginterferon/ribavirin, whereupon it is noticeable that the incidence of rash was comparable between telaprevir and placebo-treated patients.
Severe cutaneous adverse reaction
A systematic retrospective assessment by expert dermatologists was made of all 221 Grade 3 rash events, rash events leading to discontinuation of any study drugs, or rash serious AEs occurring in Phase III telaprevir trials [36]. In total, 208 (94%) of these cases were reported in patients receiving telaprevir-based therapy (N =
= 1257) [28], [29]. This assessment revealed 13 patients receiving a telaprevir-based regimen who presented with a suspected severe cutaneous adverse reaction (SCAR). Three cases of Stevens–Johnson Syndrome (SJS, 1 definite, 1 probable, and 1 possible) and 11 cases of drug reaction with eosinophillia with systemic symptoms (DRESS, 1 definite, 2 probable, 8 possible) were reported (in one patient, both diagnoses were suspected) [36]. Among the three SJS cases, one occurred 11
1257) [28], [29]. This assessment revealed 13 patients receiving a telaprevir-based regimen who presented with a suspected severe cutaneous adverse reaction (SCAR). Three cases of Stevens–Johnson Syndrome (SJS, 1 definite, 1 probable, and 1 possible) and 11 cases of drug reaction with eosinophillia with systemic symptoms (DRESS, 1 definite, 2 probable, 8 possible) were reported (in one patient, both diagnoses were suspected) [36]. Among the three SJS cases, one occurred 11 weeks after telaprevir discontinuation and was not considered related to telaprevir. Of the two suspected SJS cases that occurred during the telaprevir treatment phase, one was considered by the expert dermatologists as possible SJS, and the other as probable SJS. Among the 11 suspected cases of DRESS, three were confirmed [36]. One of these DRESS cases has been reported separately and is shown in Fig. 2 [38]. All cases of reported SJS resolved, 10 cases of reported DRESS resolved, 1 patient was lost to follow-up.
weeks after telaprevir discontinuation and was not considered related to telaprevir. Of the two suspected SJS cases that occurred during the telaprevir treatment phase, one was considered by the expert dermatologists as possible SJS, and the other as probable SJS. Among the 11 suspected cases of DRESS, three were confirmed [36]. One of these DRESS cases has been reported separately and is shown in Fig. 2 [38]. All cases of reported SJS resolved, 10 cases of reported DRESS resolved, 1 patient was lost to follow-up.
SJS (and its more severe form, toxic epidermal necrolysis [TEN]) and DRESS have a very different presentation but also a different degree of severity. SJS and TEN are very acute events, with a mortality rate of 25% during hospitalization [39], [40]. The rate of mortality for SJS is estimated to be 13%, with a mortality rate of 39% for TEN [41] depending on the SCORTEN severity score [42]. DRESS is more progressive and less severe with a mortality of around 10% [39], [40], [41], [42], [43]. Both reactions require an early diagnosis for proper management, which includes discontinuation of treatment (although the need for urgent diagnosis is more acute with SJS/TEN). Precise documentation and research of risk factors is also needed to adequately quantify and minimize the risk posed.
Rash management plan
The rate of discontinuation of all study drugs as a result of cutaneous AEs was lower in telaprevir Phase III trials than in Phase II trials, [36] following incorporation of a rash management plan into the study protocols (Table 4) [28], [29]. Although a rash management plan was implemented during the ongoing Phase II trials, the majority of patients had already completed the telaprevir dosing period by this time. All patients in Phase III trials, however, were treated following the implementation of the rash management plan at the beginning of the trials.
Table 4. Discontinuation of all study drugs resulting from rash (SSC) AEs in Phase II and Phase III clinical trials of telaprevir (overall treatment phase) [36].

* Discontinuation based on discontinuation of peginterferon, since per-protocol patients had to discontinue all other drugs if peginterferon was discontinued.
The rash management plan outlined in the Phase III trial protocols provides clear guidance for HCV-treating physicians on how to classify (Table 3) and manage rash events, with the objective of minimizing the impact of cutaneous reactions while enabling continuation of antiviral therapy where possible [28], [29]. Grade 1 or 2 (mild or moderate) rash does not require treatment discontinuation, and can be primarily treated using emollients/moisturizers and topical corticosteroids. Permitted topical or systemic antihistaminic (including diphenhydramine, hydroxyzine, levocetirizine, and desloratadine) drugs may also be used, based on local prescribing guidelines. Regular follow up is important, with advice to the patient to limit exposure to sun/heat and wear loose-fitting clothes. Grade 3 rash requires immediate discontinuation of telaprevir. Symptomatic treatment as above may also be employed. Ribavirin interruption (with or without peginterferon) is required within 7 days of stopping telaprevir if the Grade 3 rash does not improve, or sooner if it worsens [28], [29].
days of stopping telaprevir if the Grade 3 rash does not improve, or sooner if it worsens [28], [29].
However, in case of any reasonable suspicion or diagnosis of SJS, TEN, DRESS (also known as drug-induced hypersensitivity syndrome [DHS] or drug-induced delayed multiorgan hypersensitivity), acute generalized exanthematous pustulosis (AGEP), or a skin rash that is considered life-threatening, patients in Phase III telaprevir trials were required to immediately and permanently discontinue all medication [28], [29].
Perspectives on practical guidance for management of dermatological adverse events with telaprevir
The authors reviewed the available clinical trial data on telaprevir-related dermatological AEs and strategies for their management, with the aim of providing practical guidance for HCV-treating physicians. The key conclusions are presented here. These recommendations seek to allow the physician and patient the best chance of eradicating HCV, enabling them to recognize and respond appropriately to serious dermatological events while optimizing the likelihood for viral clearance with telaprevir-based therapy. Furthermore, to avoid exposing patients to the risk of severe drug-induced cutaneous reaction, HCV-treating physicians should be able to distinguish between usual dermatitis and SCAR.

Good skin care practice
In the case of Grade 1 or 2 dermatitis, patients may benefit from guidance on optimal skin care techniques that could mitigate skin symptoms and allow optimal antiviral therapy to be maintained for as long as possible. Emollient creams and lipid-rich lotions, rather than aqueous lotions or ointments, are effective and well-accepted by patients and should be prescribed as prophylactic baseline skin treatment. The patient should be instructed that proper skin care requires at least 15 min and should become a daily habit in order to become effective. This is best performed immediately after a shower or bath, when the skin is still hydrated. Application of the emollient should begin with the hands, feet, and the skin around the large joints, followed by the large skin surfaces of the trunk and extremities, and end with the neck, face, and skin folds. If required, class 3 potent topical corticosteroids can be used. Dosage can be measured by the ‘fingertip’ rule: one fingertip of cream equates to about 0.5
min and should become a daily habit in order to become effective. This is best performed immediately after a shower or bath, when the skin is still hydrated. Application of the emollient should begin with the hands, feet, and the skin around the large joints, followed by the large skin surfaces of the trunk and extremities, and end with the neck, face, and skin folds. If required, class 3 potent topical corticosteroids can be used. Dosage can be measured by the ‘fingertip’ rule: one fingertip of cream equates to about 0.5 g, sufficient to treat an area equivalent to two palms. By assessing the affected skin surface by units of palm surfaces, the therapist can accurately dose the required amount of topical corticosteroid required for a given treatment interval. Fig. 3 illustrates the basic principles of topical steroid dosing. Topical calcineurin inhibitors such as tacrolimus are not currently indicated, as they may yield high serum levels when skin barrier function is impaired.
g, sufficient to treat an area equivalent to two palms. By assessing the affected skin surface by units of palm surfaces, the therapist can accurately dose the required amount of topical corticosteroid required for a given treatment interval. Fig. 3 illustrates the basic principles of topical steroid dosing. Topical calcineurin inhibitors such as tacrolimus are not currently indicated, as they may yield high serum levels when skin barrier function is impaired.

Fig. 3. Guidance on the efficient administration of a topical steroid: the fingertip rule. (A) One fingertip of cream equates to around a 0.5g steroid dose, (B) sufficient to treat an area equivalent to two palms. By assessing the affected skin surface by units of palm surfaces, the amount of topical treatment required for a given treatment interval can be accurately assessed.
Recognition and classification of skin eruptions with telaprevir
Dermatological manifestations with telaprevir-based therapy can be considered to constitute two conditions. The large majority of cutaneous reactions represent a single dermatitis entity. This telaprevir-related dermatitis generally begins during the first 4 weeks of therapy, but can occur at any time during treatment. This eczematous dermatitis reaction is similar to reactions observed with peginterferon/ribavirin, but occurs with increased frequency and severity. Typical features of such HCV treatment-associated rash also include pruritus and skin dryness, and it is stable or slow to progress. Continuation of telaprevir together with peginterferon/ribavirin treatment is possible in Grade 1 or 2 (mild or moderate) cases, or Grade 3 cases with appropriate management (see below). In contrast, a small remainder of cases can be classified as SCAR, which is typically rare but potentially life-threatening if unrecognized or unmanaged, mandating immediate treatment discontinuation.
weeks of therapy, but can occur at any time during treatment. This eczematous dermatitis reaction is similar to reactions observed with peginterferon/ribavirin, but occurs with increased frequency and severity. Typical features of such HCV treatment-associated rash also include pruritus and skin dryness, and it is stable or slow to progress. Continuation of telaprevir together with peginterferon/ribavirin treatment is possible in Grade 1 or 2 (mild or moderate) cases, or Grade 3 cases with appropriate management (see below). In contrast, a small remainder of cases can be classified as SCAR, which is typically rare but potentially life-threatening if unrecognized or unmanaged, mandating immediate treatment discontinuation.
Management of grades 1–3 telaprevir-associated dermatitis
In line with the rash management plan implemented in Phase III trials, the telaprevir prescribing information stipulates that Grade 1 and 2 dermatological reactions to telaprevir do not require treatment interruption, but that Grade 3 reactions require telaprevir discontinuation followed by ribavirin and/or peginterferon discontinuation within 7 days if the reaction does not improve, or sooner if it worsens [27].
days if the reaction does not improve, or sooner if it worsens [27].
In some cases, Grade 3 dermatitis reactions affecting more than 50% of body surface area but with no signs of SJS, TEN, DRESS, EM or AGEP may be manageable using topical corticosteroids without treatment discontinuation. In such cases, however, hospitalization of the patient is required, and experienced dermatologists should be responsible for patient management and close follow up for signs of progression. It is important for physicians to be aware of the prescribing information for telaprevir and local guidelines for management of dermatological adverse drug reactions.
Appropriate guidelines, as evidenced from the Phase III studies of telaprevir (Table 3), permit the continuation of peginterferon/ribavirin treatment after the cessation of telaprevir in order to optimize the chance of SVR while minimizing the risk of DRESS or SJS. The less common but potentially life-threatening reactions such as SJS, TEN, and DRESS require cessation of all treatment.
AGEP is generally characterized by an acute, widespread edematous erythema with the presence of small non-follicular pustulosis mostly in the folds and the face, and is associated with elevated neutrophils and high fever [40], [44]. The reaction lasts for a few days. While EM is not a life-threatening reaction, there has been some historical confusion between EM and the separate entity of SJS [39], [45]. Ensuring the correct diagnosis is made and appropriate action is taken is therefore important when considering discontinuing antiviral treatment. While SJS is drug-induced, EM usually occurs post-infection and is characterized by typical target lesions, chiefly on the extremities, rather than the widespread macules or blisters associated with SJS [39]. Target lesions are defined as less than 3 cm in diameter, with at least 3 ‘zones’: a central zone of dusky erythema or purpura (sometimes blistering), a middle paler area of oedema, and a well-defined outer ring of erythema [46]. All other target lesions lacking this pattern of three zones should be considered atypical target lesions. In cases of suspicion of EM, we would advise that telaprevir discontinuation should be considered, and implemented if the reaction appears different to the general dermatitis reaction, gives rise to any reasonable suspicion of SJS/TEN or DRESS, or progresses in severity.
cm in diameter, with at least 3 ‘zones’: a central zone of dusky erythema or purpura (sometimes blistering), a middle paler area of oedema, and a well-defined outer ring of erythema [46]. All other target lesions lacking this pattern of three zones should be considered atypical target lesions. In cases of suspicion of EM, we would advise that telaprevir discontinuation should be considered, and implemented if the reaction appears different to the general dermatitis reaction, gives rise to any reasonable suspicion of SJS/TEN or DRESS, or progresses in severity.
The severity of telaprevir-associated dermatitis events dictates the frequency of evaluation by the HCV-treating physician. In the case of a Grade 1 event, it is recommended that the patient should be re-evaluated between days 2 and 4 after the onset of rash. Patients with a Grade 2 event should be seen at day 2. Grade 3 events require follow up on days 1, 3, and 7. Additional regular follow-up of patients is required until the reaction is completely resolved.
Guidance for distinguishing between telaprevir-related dermatitis and SCAR
In accordance with the Phase III rash management plan, and in contrast to the telaprevir-related dermatitis, SJS, TEN, DRESS, EM, and AGEP reactions require immediate discontinuation of all treatment (telaprevir, peginterferon, and ribavirin) and referral to a dermatologist. A number of clinical and biological signs and symptoms have been identified from the clinical trial database that may help HCV-treating physicians to distinguish between telaprevir-related dermatitis, where antiviral treatment can often be continued and supportive treatment given, and the less common but potentially more harmful SJS and DRESS reactions. These are illustrated in the algorithm in Fig. 4 and outlined below. (Click to enlarge)

Fig. 4. Algorithm for distinguishing between telaprevir-related dermatitis and SCAR in a rapidly progressing skin reaction. DRESS: drug reaction with eosinophilia and systemic symptoms (also known as drug-induced hypersensitivity syndrome); SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis; ALT, alanine transaminase.
Patients with a rash that appears to be unlike the telaprevir-associated dermatitis should be assessed for signs that may indicate possible DRESS. Criteria that should alert the physician include onset from 5 to 10 weeks after first dose, rapidly progressing skin rash, prolonged fever (>38.5
weeks after first dose, rapidly progressing skin rash, prolonged fever (>38.5 °C), and facial edema. If any of these signs are present, the patient should be urgently examined for the following ‘confirmation criteria’: enlarged lymph nodes, eosinophilia, atypical lymphocytes, and rise in alanine transaminase, alkaline phosphatase or creatinine. If any confirmation criteria are found, telaprevir, peginterferon, and ribavirin treatment should be discontinued immediately and permanently, and the patient referred to a dermatologist. It is important to note that there is a greater urgency for prompt diagnosis and appropriate action for SJS and TEN. Patients presenting with mucosal involvement of at least two sites, or with blisters or epidermal detachment (at sites beyond the site of peginterferon injection) should immediately and permanently discontinue telaprevir, peginterferon, and ribavirin and be referred to a dermatologist. Rapidly progressing skin rash, skin pain, and atypical or typical target lesions may also be present in cases of SJS or TEN and should alert the physician to assess the patient for mucosal involvement, blisters or positive Nikolsky signs (epidermal detachment under lateral pressure on erythema).
°C), and facial edema. If any of these signs are present, the patient should be urgently examined for the following ‘confirmation criteria’: enlarged lymph nodes, eosinophilia, atypical lymphocytes, and rise in alanine transaminase, alkaline phosphatase or creatinine. If any confirmation criteria are found, telaprevir, peginterferon, and ribavirin treatment should be discontinued immediately and permanently, and the patient referred to a dermatologist. It is important to note that there is a greater urgency for prompt diagnosis and appropriate action for SJS and TEN. Patients presenting with mucosal involvement of at least two sites, or with blisters or epidermal detachment (at sites beyond the site of peginterferon injection) should immediately and permanently discontinue telaprevir, peginterferon, and ribavirin and be referred to a dermatologist. Rapidly progressing skin rash, skin pain, and atypical or typical target lesions may also be present in cases of SJS or TEN and should alert the physician to assess the patient for mucosal involvement, blisters or positive Nikolsky signs (epidermal detachment under lateral pressure on erythema).
Summary and conclusions
HCV and its treatment with peginterferon/ribavirin are associated with significant dermatological complications. In the era of DAA-based triple combination therapy, however, management of dermatological AEs will become an even more important consideration for HCV-treating physicians. Effective management strategies will be of great importance in limiting the severity and impact of dermatological side effects on treatment outcomes.
The majority of cutaneous AEs occurring with telaprevir can be classified as a less harmful eczematous dermatitis, associated with pruritus and xerosis. Most cases of this dermatitis reaction are mild or moderate, in which case good skin care practice, coupled with vigilance for the rare signs of more serious reactions, should enable antiviral therapy (peginterferon/ribavirin with or without telaprevir) to be maintained in order to increase the chances of patients achieving an SVR. Rare cases of severe cutaneous reactions including DRESS and SJS have been reported and resolved upon antiviral treatment discontinuation. Even though these cases are rare, special attention to skin symptoms occurring during HCV treatment and strict adherence to the rash management plan is required in order to detect severe cutaneous reactions as early as possible.
Conflict of interest
Patrice Cacoub has been a consultant and invited speaker for Schering Plough, Roche Pharma, Janssen Pharmaceuticals, Servier, Vifor Pharma, Sanofi-Aventis, Pfizer, and has received educational grants from Schering Plough, Gilead, Servier, Vifor Pharma, Glaxo SmithKline; Marc Bourlière has been a consultant and invited speaker for Janssen Pharmaceuticals, Roche Pharma, Schering Plough, Merck, Gilead, BMS, Novartis, and GlaxoSmithKline; Jann Lübbe has received speaker honoraria from Janssen Pharmaceuticals and Roche Pharma; Nicolas Dupin has been a consultant for Janssen Phamaceuticals and Boehringer Ingelheim; Peter Buggisch has been a consultant and invited speaker for Janssen Pharmaceuticals, Roche Pharma, Schering Plough, Merck, Gilead and Novartis; Geoffrey Dusheiko has received consulting fees from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Human Genome Sciences, Novartis, Pharmasset, Pfizer, Roche–Genentech, Schering-Plough (Merck), Tibotec, Vertex Pharmaceuticals, and ZymoGenetics, travel support from Gilead Sciences, and grant support from Gilead Sciences, Novartis, Pharmasset, Hoffmann–La Roche, Schering-Plough (Merck), Tibotec, and Vertex Pharmaceuticals; Christophe Hézode has been a consultant and invited speaker for Janssen Pharmaceuticals; Odile Picard has been an invited speaker for Janssen Pharmaceuticals; Ramon Pujol has been a consultant for Janssen Pharmaceuticals; Siegfried Segaert has been a consultant and invited speaker for Janssen Pharmaceuticals; Bing Thio has been a consultant and invited speaker for Janssen Pharmaceuticals and has received an educational grant from Janssen Pharmaceuticals; Jean-Claude Roujeau has been a consultant and invited speaker for Boehringer Ingelheim, Janssen Pharmaceuticals, Johnson & Johnson, Medimmune, OM Pharma, Pfizer, Servier, Vertex and has received research grants from Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Sanofi-Aventis, Servier and Wyeth.
This clinical review reflects the detailed discussion and opinions of the authors on data and literature reviewed at an advisory board meeting on the dermatological manifestations of HCV treatments held in Paris, France in March 2011. The advisory board meeting was sponsored by Janssen Pharmaceuticals, however the content of this paper does not necessarily reflect the opinions of the meeting sponsor. Medical writing support was provided by Tom Westgate of Gardiner-Caldwell Communications (funded by Janssen Pharmaceuticals), who developed the first draft of the manuscript based on the authors’ recommendations of relevant published papers and the debate and discussion during the meeting. All authors substantially contributed to development of all drafts of the manuscript and have read and approved the final draft. The corresponding author had full access to the source literature and takes full responsibility for the content of the paper and for the decision to submit.
References
Source












