Journal of Gastroenterology and Hepatology
Special Issue: Third Asian-Pacific Topic Conference (APTC2012): Nutrition-related disorders and digestive system. Organized by Japanese Society of Gastroenterology (JSGE) and Asian-Pacific Association of Gastroenterology (APAGE), Tokyo, Japan, November 2–3, 2012. Guest Editor: Soichiro Miura
Volume 28, Issue Supplement S4, pages 93–98, December 2013
Nutrition-Related Liver Disorders: NAFLD
You have free access to this content
Keisuke Hino*, Sohji Nishina, Yuichi Hara
Article first published online: 19 NOV 2013
DOI: 10.1111/jgh.12243
© 2013 Journal of Gastroenterology and Hepatology Foundation and Wiley Publishing Asia Pty Ltd
Keywords: hepatitis C; hepcidin; iron; oxidative stress; reactive oxygen species
Abstract
The liver is the major iron storage organ in the body, and therefore, iron metabolic disorder is sometimes involved in chronic liver diseases. Chronic hepatitis C is one of the liver diseases that show hepatic iron accumulation, even though its level should be recognized to be basically mild to moderate and sometimes within the normal range. The mechanisms underlying hepatic iron accumulation in chronic hepatitis C have not been fully elucidated. Reduction of the hepcidin transcription activity by hepatitis C virus (HCV)-induced reactive oxygen species may in part account for it, but the regulation of hepcidin is very complex and may depend on many variables, including the particular stage of the systemic and/or hepatic inflammatory conditions and the circulating transferrin-bound iron and intracellular iron stores. This might explain the variations in hepatic iron concentrations reported among patients with HCV-related chronic liver disease. However, even mild-to-moderate iron overload in the liver contributes to disease progression and hepatocarcinogenesis in chronic hepatitis C probably by reinforcing the HCV-induced oxidative stress through Fenton reaction. The present review highlights the current concept of hepatic iron overload status in chronic hepatitis C and discusses how iron metabolic disorder develops in this disease and the impact of hepatic iron overload on disease progression and its relevance to hepatocarcinogenesis.
Introduction
Approximately 170 million people worldwide are infected with hepatitis C virus (HCV).[1] HCV infection often remains asymptomatic but can lead to severe liver damage. However, how HCV causes liver injury and liver cancer is not fully understood. Histological examination has revealed that chronic inflammation seems to play an important role in the pathogenesis of chronic hepatitis C, and excess iron also is associated with increased morbidity and mortality.[2, 3] In addition, a study using electron microscopy and X-ray microanalysis demonstrated that almost all liver specimens from patients with chronic hepatitis C had at least some lysosomal iron deposits even when no iron deposit was evident with standard optical microscopy and Prussian Blue staining.[4] Elevated iron-related serum markers and increased hepatic iron accumulation are relatively common and correlate with the severity of hepatic inflammation and fibrosis in patients with chronic hepatitis C. Excess divalent iron can be highly toxic mainly via the Fenton reaction producing hydroxyl radicals.[5] This is particularly relevant for chronic hepatitis C, in which oxidative stress has been proposed as a major mechanism of liver injury. Oxidative stress and increased iron levels strongly favor DNA damage, genetic instability, and tumorgenesis. Indeed, a significant correlation between 8-hydroxy-2'-deoxyguanosine (8-OHdG), a marker of oxidatively generated DNA damage,[6] and hepatic iron excess has been shown in patients with chronic hepatitis C.[7] Kato et al. reported that phlebotomy lowered the risk of progression to hepatocellular carcinoma (HCC),[8, 9] which showed the critical role of iron in the development of HCC in patients with chronic hepatitis C. Thus, there is a critical interaction between HCV infection and hepatic iron overload in the progression of liver disease and the development of HCV-related HCC. However, the mechanisms underlying hepatic iron overload and its contribution to hepatocarcinogenesis in chronic hepatitis C are not fully elucidated. The present review highlights the current concept of hepatic iron overload status in chronic hepatitis C and discusses how iron metabolic disorder develops in chronic hepatitis C, the impact of hepatic iron overload on disease progression, and its relevance to hepatocarcinogenesis.
Regulation of systemic iron homeostasis
In normal adults, storage iron is deposited in hepatocytes and tissue macrophages and mobilized in response to acute need. Serum iron levels are determined both by intestinal absorption and macrophage recycling of iron from hemoglobin because there is no efficient pathway for iron excretion.[10] Regulatory effectors that modulate intestinal iron absorption probably also modulate the release of iron from tissue macrophages and hepatocytes. Hepcidin appears to be such a regulatory effector. It is a small, cysteine-rich peptide, cleaved from a larger precursor.[11-13] Hepcidin, which was originally isolated from human serum and urine as a peptide with antimicrobial activity,[11, 13] is a hormone exclusively synthesized in the liver and a soluble regulator that acts to attenuate both intestinal iron absorption and iron release from reticuloendothelial macrophages.[12, 14] Increased plasma iron from macrophage recycling of aged red blood cells or from intestinal absorption of iron stimulates hepatocytes through several signaling pathways to produce more hepcidin. Ferroportin is an iron exporter on the surface of absorptive intestinal enterocytes, macrophages, hepatocytes, and placental cells, all of which release iron into plasma.[15-17] Circulating hepcidin can bind to ferroportin, cause internalization, and trap iron in hepatocytes, macrophages, and absorptive enterocytes.[18] Thus, coupling the internalization of ferroportin to hepcidin levels generates a homeostatic loop regulating the iron plasma level and the tissue distribution of iron.
Transcriptional regulation of hepcidin
Knowledge of how hepcidin transcription is regulated within hepatocytes appears to be indispensable for understanding the mechanisms underlying hepatic iron overload in chronic hepatitis C because hepcidin is the central regulator of systemic iron homeostasis. Important elements of the signaling pathway present on the hepatic plasma membrane that affect hepcidin transcription include transferrin receptor 2 (TfR2),[19] HFE,[20] which is the protein affected in the most common form of genetic hemochromatosis, and hemojuverin (HJV),[21] a member of the bone morphogenetic protein (BMP) receptor family. The mechanisms by which TfR2, HFE, and HJV are linked to changes in hepcidin transcription are incompletely understood, but the discovery of HJV revealed that the well-known sons of mothers against decapentaplegic (SMAD) signal transduction pathway was important in this process.[22] Notably, animals that lack hepatocyte SMAD4, a protein that combines with other members of the SMAD family to regulate transcription of target genes, develop significant iron overload associated with a profound reduction in hepcidin expression.[23] Interleukin 6 (IL-6) activates hepcidin transcription through a pathway that involves janus kinase-signal transducer and activator of transcription (STAT) signaling and a binding site for the transcription factor STAT3.[24, 25] The transcription factor CCAAT/enhancer-binding protein α (C/EBPα) is also clearly involved in regulating hepcidin transcription.[26] C/EBPα knockout mice demonstrate decreased hepcidin expression and iron overload.[26]
The pathways described earlier activate hepcidin transcription, but only one pathway has been identified that represses hepcidin expression. The transmembrane serine protease (TMPRSS6) is part of the pathway that suppresses hepcidin expression as revealed in TMPRSS6 mutant mice.[27]
Hepatic iron accumulation in chronic hepatitis C
Based on the assumption that one-third of iron stores are normally in the liver, this would translate to a normal median hepatic iron content of 0.27 g for men and 0.13 g for women.[28] Extensive studies reported median hepatic iron concentrations of 396 (range 0–2105) and 458 (range 114–2190) μg/g dry weight liver tissue in patients with chronic hepatitis C.[29, 30] These results suggest that hepatic iron content in patients with chronic hepatitis C is approximately 0.50∼0.69 g, equivalent to two to five times the normal hepatic iron content if the liver weight is estimated to be 1500 g. In contrast, a hepatic iron index (μmol Fe/g liver tissue/patients age) of 1.9 or more has been reported to be typical of patients with hereditary hemochromatosis.[31] If the hepatic iron index of a patient aged 60 with hereditary hemochromatosis is 1.9, the hepatic iron concentration of this patient is assumed to be 6384 μg/g liver tissue. Thus, we should understand that hepatic iron content is much less in chronic hepatitis C than in hereditary hemochromatosis, even though it is recognized to be one of liver diseases that show hepatic iron accumulation.
There also remains uncertainty as to whether iron predominantly accumulates in hepatocytes or the reticuloendothelial system, mainly Kupffer cells, in patients with chronic hepatitis C. Some clinical studies showed that iron was mainly localized in the reticuloendothelial system,[32, 33] whereas others reported its localization in hepatocytes.[34] Interestingly, Fiel et al. documented that even ribavirin-associated hemolysis deposited iron preferentially in hepatocytes in patients with chronic hepatitis C.[35] Hepatocytic iron accumulation may indicate potential DNA damage and genetic instability in association with HCV-induced oxidative stress, whereas iron deposition in Kupffer cells may contribute to cytokine release leading to inflammation or fibrosis. However, further investigations are needed to clarify this issue.\
Mechanisms underlying hepatic iron accumulation in chronic hepatitis C
HFE is a major histocompatibility class I-like (MHC) molecule that, unlike other known classical and non-classical MHC proteins, has a regulatory role in the functions of iron metabolism in cells and the body. A homozygous mutation in the HFE protein in humans that changes cysteine at position 282 to tyrosine is responsible for iron overload and organ damage resulting in hemochromatosis.[36] The role of HFE mutations in chronic hepatitis C has been well reviewed.[37] In general, patients with chronic hepatitis C seem to have no difference in the prevalence of heterozygosity for HFE mutations as compared with a control population. It is still controversial as to whether HFE mutations are associated with hepatic iron overload in chronic hepatitis C probably because of the different methodologies used to measure hepatic iron and/or confounding variables such as demographic parameters, environmental factors, hepatic inflammatory activity, and the duration of HCV infection among the reported studies. In addition, HFE mutations are seemingly not associated with the progression of liver disease in chronic hepatitis C patients even though HFE may affect Kupffer cells or interact with immune cells.
Fujita et al. showed for the first time that hepatic hepcidin messenger RNA (mRNA) levels adjusted by serum ferritin values were significantly lower in patients with chronic hepatitis C than in those with chronic hepatitis B or those without hepatitis B virus (HBV) or HCV infection.[38] Of note, the relative expression of hepcidin for iron stores was lower in chronic hepatitis C than in chronic hepatitis B or chronic liver diseases without HBV or HCV infection, even though hepcidin expression levels were strongly correlated with serum ferritin and the degree of hepatic iron deposition. These results suggested that hepcidin might play a pivotal role in iron overload in patients with chronic hepatitis C. A recent study using a validated immunoassay of the 25 amino acid bioactive hepcidin in serum also revealed that serum hepcidin levels were lower in patients with chronic hepatitis C than in controls despite a significant correlation between hepcidin and serum ferritin or the histological iron score in both groups.[39] Thus, the relatively decreased synthesis of hepcidin in chronic hepatitis C contrasts with the absolute deficit or lack in hepcidin synthesis observed in hereditary hemochromatosis and may account for the mild-to-moderate hepatic iron overload observed in some patients with chronic hepatitis C.
Regulation of hepcidin transcription by HCV, iron overload, and inflammation
The next question is how hepcidin transcription is suppressed in the presence of HCV infection. Which pathway for regulating hepcidin transcription is affected? Oxidative stress is present in chronic hepatitis C to a greater degree than in other inflammatory liver diseases.[32] The HCV core protein induces the production of reactive oxygen species (ROS) through inhibition of mitochondrial electron transport.[40] Interestingly, alcohol metabolism-mediated ROS were shown to suppress hepcidin transcription via C/EBPα.[41] Therefore, we investigated the mechanisms underlying hepcidin transcription inhibited by HCV focusing on ROS production, which plays a critical role in the pathogenesis of both alcoholic liver disease and chronic hepatitis C. Hepcidin promoter activity and the DNA binding activity of C/EBPα were downregulated concomitant with increased expression of C/EBP homology protein, an inhibitor of C/EBP DNA binding activity, and with increased levels of ROS in transgenic mice expressing the HCV polyprotein[42] (Fig. 1). Thus, the mechanisms underlying HCV-related hepatic iron overload appear to have some similarities with alcohol-induced iron overload in terms of disrupted hepcidin transcription through suppressed activity of C/EBPα. In agreement with our observation, an in vitro study by Miura et al. using hepatoma cells showed that HCV-induced ROS inhibited the binding activity of C/EBPα to the hepcidin promoter through increased histone deacetylase activity.[43]
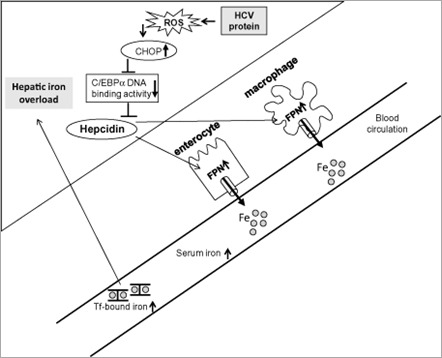
Figure 1. Schematic diagram depicting the mechanisms underlying the hepatic iron accumulation in transgenic mice expressing the hepatitis C virus (HCV) polyprotein. HCV protein-induced reactive oxygen species (ROS) increase hepatic expression of CCAAT/enhancer-binding protein (C/EBP) homology protein (CHOP) and subsequently reduce DNA binding activity of C/EBPα, which leads to reduction of hepcidin transcription. Decreased hepcidin expression increases ferroportin (FPN) expression in the enterocytes and reticuloendothelial macrophages resulting in increased duodenal iron transport and macrophage iron release, which lead to hepatic iron accumulation.
Hepcidin is also regulated by both circulating transferrin-bound iron and intracellular iron stores. The exact mechanism is still unknown but seems to involve the BMP/SMAD pathway. As yet, there is no convincing evidence that accounts for the suppressive transcription of hepcidin through the BMP/SMAD cascade in chronic hepatitis C. Taking into account the significant correlation between hepcidin and serum ferritin, or the histological iron score, hepcidin transcription seems to be properly regulated in response to the iron concentration in chronic hepatitis C. Thus, the opposing effects of HCV-induced hepcidin-suppressive factors and iron load-induced hepcidin-stimulation factors potentially regulate hepcidin transcription in chronic hepatitis C. As suggested by Girelli et al.,[39] in the early phase of chronic hepatitis C hepcidin may be prominently suppressed by HCV, but as iron accumulates, the negative influence of viral factors may be masked by the positive stimulation of iron.
Inflammation also regulates hepcidin transcription. Pro-inflammatory cytokines such as IL-6 mediate this response by inducing transcription of hepcidin mRNA via STAT3, which binds to a STAT-responsive element within the hepcidin promoter.[24, 25] Our transgenic mice expressing the HCV polyprotein did not show any inflammation in the liver. A possible pitfall in this experimental model was that we could not take the inflammatory effect on hepcidin regulation into account, which is different from what is observed in patients with chronic hepatitis C. Serum levels of IL-6 have been shown to be elevated in patients with HCV-related chronic liver disease,[44] which raises the possibility that IL-6 acts to stimulate hepcidin expression through the STAT3 pathway. This would be expected to counteract the decrease in hepcidin transcription caused by HCV-induced ROS. However, no significant relationship has been found between serum IL-6 and hepcidin in patients with chronic hepatitis C,[39, 45] even though a paracrine effect of local IL-6 release on hepcidin transcription in the liver cannot be excluded. On the other hand, chronic inflammation with production of pro-inflammatory cytokines has the potential to deliver an additional burden of ROS, which would be expected to reinforce the decrease in hepcidin transcription. Most likely, during chronic inflammation states in vivo like chronic hepatitis C, the regulation of hepcidin is more complex and may depend on many variables, including the particular stage of systemic and/or hepatic inflammatory disease. This might explain the variations in hepatic iron concentrations reported among patients with HCV-related chronic liver disease. The schematic outline in Figure 2 depicts the assumed mechanisms underlying the hepatic iron accumulation in chronic hepatitis C.
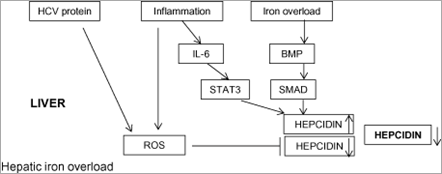
Figure 2. Schematic diagram depicting the assumed mechanisms underlying the hepatic iron accumulation in patients with chronic hepatitis C. Hepcidin transcription in chronic hepatitis C may be potentially regulated by the opposing effects of hepatitis C virus (HCV)-related reactive oxygen species (ROS)-induced hepcidin suppression and iron load-induced hepcidin stimulation. Inflammation may also have the opposing effects of stimulation and suppression of hepcidin transcription through the interleukin (IL)-6/signal transducer and activator of transcription (STAT) pathway and ROS pathway, respectively. Consequent relative suppression of hepcidin expression is potentially one of the mechanisms underlying the hepatic iron accumulation in patients with chronic hepatitis C. BMP, bone morphogenetic protein; SMAD, sons of mothers against decapentaplegic.
Relevance of hepatic iron overload to hepatocarcinogenesis
Studies in HCV-infected and uninfected chimpanzees demonstrated that iron loading did exacerbate liver injury in HCV-infected chimpanzees and that HCV infection increased the susceptibility of the liver to injury following iron loading.[46] Increased hepatic iron deposition is reported to be associated with more advanced liver fibrosis in patients with chronic hepatitis C.[47] Recently, it has been prospectively shown in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis Trial cohort that stainable iron in hepatocytes and portal tract cells predicts progression and outcomes (Child–Pugh score > 7, ascites, encephalopathy, variceal bleeding, spontaneous bacterial peritonitis, HCC, and death) in advanced chronic hepatitis C.[48] Thus, iron is a cofactor that influences the severity and progression of chronic hepatitis C.
Although the association of markedly increased iron accumulation in the liver with hepatocarcinogenesis in hereditary hemochromatosis has been well described,[49] it remains to be elucidated whether mild-to-moderate increases in hepatic iron accumulation contribute to the development of HCC in patients with HCV-associated chronic liver diseases. Nevertheless, there are several lines of evidence that suggest the association of hepatic iron overload with hepatocarcinogenesis in chronic hepatitis C. It has been reported that hepatic iron storage is strongly correlated with hepatic 8-OHdG levels and that subsequent oxidative DNA damage in the liver is associated with an increased risk of HCC development.[2] In addition, the decrease in hepatic 8-OHdG content caused by phlebotomy lowers the risk of progression to HCC, which indeed shows the critical role of the iron-overload state in the development of HCC in patients with chronic hepatitis C.[8, 9]
We investigated whether mild iron overload actually induced HCC in the presence of HCV protein using transgenic mice expressing the HCV polyprotein. Transgenic mice fed an excess-iron diet showed marked hepatic steatosis, including the centrilobular microvesicular type, ultrastructural alterations of the mitochondria and decreased degradation activity of fatty acid at 6 months, as well as hepatic accumulation of lipid peroxidation products and 8-OHdG at 12 months after the initiation of feeding. Of note, hepatic tumors including HCC developed in 5 of 11 (45%) transgenic mice fed the excess-iron diet at 12 months after the initiation of feeding but did not in control mice or transgenic mice fed the control diet.[50] These results indicate the importance of oxidative stress and subsequent mitochondrial injury synergistically induced by iron loading and HCV proteins in the development of HCC. Thus, there seems to be a close relationship between the development of HCC and oxidative DNA damage synergistically induced by hepatic iron accumulation and HCV proteins. However, further investigations are needed to clarify the detailed mechanisms by which hepatic iron accumulation results in the development of HCC in chronic hepatitis C.
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research (B) (23390201) from the Japan Society for the Promotion of Science, by a Health and Labor Sciences Research Grant for Research on Hepatitis from the Ministry of Health, Labor and Welfare of Japan and by a Research Project Grant P2 from Kawasaki Medical School.
References
Source



