World J Gastroenterol 2013 November 14; 19(42): 7316-7326
Published online 2013 November 14. doi: 10.3748/wjg.v19.i42.7316.
Copyright ©2013 Baishideng Publishing Group Co., Limited. All rights reserved.
Marco Vivarelli, Roberto Montalti, Hepatobiliary and Abdominal Transplantation Surgery, Department of Gastroenterology and Transplantation, Polytechnic University of Marche, 60129 Ancona, Italy
Andrea Risaliti, Department of Surgery and Transplantation, University of Udine, 33100 Udine, Italy
Author contributions: Vivarelli M conceived and designed the study; Montalti R drafted the article and acquired, analyzed and interpreted the data; Risaliti A revised the manuscript.
Correspondence to: Marco Vivarelli, MD, Hepatobiliary and Abdominal Transplantation Surgery, Department of Gastroenterology and Transplantation, Polytechnic University of Marche, A.O.U. “Ospedali Riuniti”, Via Conca 71, 60129 Ancona, Italy. vivarelli63@libero.it
Telephone: +39-71-5965099 Fax: +39-71-5965100
Received July 3, 2013; Revised August 8, 2013; Accepted September 16, 2013;
Abstract
Hepatocellular carcinoma (HCC) is the most frequent primary liver tumor, and overall, it is one of the most frequent cancers. The association of HCC with chronic liver disease, and cirrhosis in particular, is well known, making treatment complex and challenging. The treatment of HCC must take into account the presence and stage of chronic liver disease, with the aim of preserving hepatic function that is often already impaired, the stage of HCC and the clinical condition of the patient. The different treatment options include surgical resection, transplantation, local ablation, chemoembolization, radioembolization and molecular targeted therapies; these treatments can be combined in various ways to achieve different goals. Ideally, liver transplantation is best treatment for early stage HCC on cirrhosis because it removes both the tumor and the chronic disease that produced it; however, the application of this powerful tool is limited by the scarcity of donors. Downstaging and bridging are different strategies for the management of HCC patients who will undergo liver transplantation. Several professionals, including gastroenterologists, radiologists and surgeons, are involved in the choice of the most appropriate treatment for a single case, and a multidisciplinary approach is necessary to optimize the outcome. The purpose of this review is to provide a comprehensive description of the current treatment options for patients with HCC by analyzing the advantages, disadvantages and rationale for their use.
Keywords: Hepatocellular carcinoma, Multimodal treatment, Locoregional treatments, Molecular targeted therapies, Liver resection, Liver transplantation
Core tip: Hepatocellular carcinoma (HCC) occurs frequently, and its association with cirrhosis makes treatment complex and challenging. The treatment of HCC must take into account the presence and stage of chronic liver disease with the aim of preserving hepatic function that is often already impaired. The different treatment options include surgical resection, transplantation, local ablation, chemoembolization, radioembolization and molecular targeted therapies. Downstaging and bridging are different strategies for the management of HCC patients who will undergo liver transplantation. The purpose of this review is to provide a comprehensive description of the current treatment options for patients with HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for nearly 90% of the primary liver tumors and is currently the third leading cause of cancer death worldwide. Approximately 500000 new cases of HCC are diagnosed worldwide each year, with a peak incidence observed in countries in which the hepatitis B virus (HBV) is endemic, such as Southeast Asia and sub-Saharan Africa[1]. As the number of carriers of chronic liver disease increases, HCC has become a major public health issue.
The main risk factor for HCC is the presence of chronic liver disease, particularly when the disease has already resulted in liver cirrhosis. The constant process of destruction and repair within the parenchyma that is associated with cirrhosis increases hepatocyte metabolism and amplifies the risk of mutations in a multistep progression from hyperplastic nodule to early HCC and finally to moderately/poorly differentiated HCC.
Hepatitis B and C viruses (HBV and HCV) are known to have oncogenic potential, and the risk of HCC in HBV and HCV carriers is increased independently of the presence of cirrhosis[2,3]. Malignant transformation of hepatocytes in the infected liver could be caused by chronic inflammation and the oxidative DNA damage that leads to genetic and epigenetic changes. There is also evidence that proteins encoded by HBV and HCV may have a direct role in hepatocarcinogenesis; in fact, proteins encoded in the genome of each of these viruses have been linked to alterations in hepatocyte physiology and hepatocellular signal transduction[4]. Other causes of chronic liver diseases, such as alcohol abuse, non-alcoholic steatohepatitis (NASH), hemochromatosis, α1-antitrypsin deficiency, autoimmune disease and Wilson disease are associated with a higher risk of developing HCC and require close patient monitoring. In Western countries, obesity and metabolic syndromes associated with type II diabetes, which are strictly related with NASH, are now emerging as new potential predisposing factors for HCC[1].
Among patients with cirrhosis, the cumulative 5-year risk of developing HCC ranges from 5% to 30%, depending on the presence and stage of underlying liver disease, ethnicity, age, sex and duration of the exposure to primary hepatotropic viruses[1,5].
The main peculiarity of HCC is that the treatment of the tumor must take into account the presence and stage of chronic liver disease, with the aim of preserving hepatic function that is often already impaired. A key step in the choice of therapy is therefore the correct assessment of the functional reserve of the liver, which is often more important than the staging of the tumor itself.
Several options are available for the treatment of HCC, and these can often be combined; the choice of treatment and the timing of its administration therefore must be balanced accurately.
The aim of the present review is to provide an update than can be useful in clinical practice for determining the most appropriate treatment for HCC patients.
HCC SCREENING
The achievement of a curative treatment for HCC depends on the detection of the tumor at an early stage. Once the population at risk is identified, screening of HCC is based on ultrasonography and measurement of serum alpha-fetoprotein (s-AFP) levels.
It is recommended that patients with advanced liver fibrosis (F3) or established cirrhosis undergo a liver ultrasound (US) and serum measurements every 6 mo[6]. In these patients, ultrasound has a sensitivity between 58% and 89% and a specificity of 90%[7]. The sensitivity of s-AFP measurements is lower and ranges between 25% and 65% when values above 20 ng/mL are considered positive[1]. The sensitivity and specificity of s-AFP measurement for the detection of HCC increase proportionally with higher blood s-AFP levels, particularly when the level is above 400 ng/mL.
Once the presence of HCC is confirmed, the s-AFP level can be correlated with tumor stage, particularly with the size and multifocality of the tumor and the presence of microvascular invasion. Overall, a screening strategy that combines abdominal ultrasonography and measurement of s-AFP every 6 mo in patients with cirrhosis can reduce HCC mortality by approximately 40%[8,9].

Figure 1 Diagnostic algorithm for hepatocellular carcinoma. Modified by Forner et al[11]. MDCT: Multidetector computed tomography; MR: Magnetic resonance; CT: Computed tomography; MRI: Magnetic resonance imaging.
The nature of nodules with a diameter of less than 1 cm cannot be precisely defined during ultrasound, and a follow-up control after 3-4 mo is required. Nodules detected at US with a diameter greater than 10 mm must be further investigated with contrast-enhanced triphasic or quadriphasic computed tomography (CT) imaging or magnetic resonance (MR) imaging. The diagnosis of HCC is based on an arterial hypervascular phase (wash-in) followed by disappearance of the contrast in the venous phase (wash-out)[12].
Recent reports have demonstrated that MR has a higher sensitivity compared with CT[13]; however, if the data from the first imaging procedure are not conclusive, confirmation using a different technique is recommended. In cases in which the diagnosis is uncertain, a s-AFP level > 400 ng/mL has a high positive predictive value[1]. Histological confirmation through percutaneous liver biopsy should be restricted to those nodules with features on MR or CT that are not typical enough to allow a diagnosis[14]. In fact, the histological diagnosis of HCC is complex, requires a great degree of expertise and relies on the assumption that the core of the nodule has been effectively sampled by the small needle that is used for the percutaneous biopsy. The sensitivity and specificity reported for nodules less than 20 mm in diameter is 60%[15]. A risk of tumor seeding in the path of the puncture has been reported in approximately 2.5% of the cases with a median time of development of 17 mo[16].
HCC staging
The severity of chronic liver disease is usually classified according to the Child-Pugh and model for end-stage liver disease (MELD) scores, and the tumor is staged with the TNM system; in the setting of liver transplantation, the indication for liver replacement is based mainly on the Milan criteria[17]. Another staging system that has gained acceptance is the Barcelona Clinic Liver Cancer (BCLC) score, the advantage of which is that it takes into account the characteristics of the tumor as well as the liver function and the general conditions of the patient[8] (Figure 2). The BCLC system was developed after a retrospective analysis of several cohort studies on patients with HCC at different stages. This system identifies patients with early HCC who may benefit from curative therapies (stage 0 and A), those at intermediate (stage B) or advanced (stage C) stages who may benefit from palliative treatments and those with a very poor life expectancy (stage D). BCLC is the most commonly used staging system in Europe, and it has been approved by the European Association for the Study of the Liver (EASL) and the AASLD.
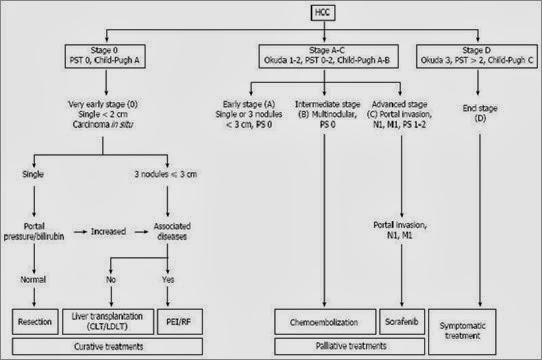
Figure 2 The Barcelona Clinic Liver Cancer staging system and treatment allocation. Copyright © 2010, American Association for the Study of Liver Diseases. CLT: Cadaveric liver transplantation; HCC: Hepatocellular carcinoma; LDLT: Living donor liver transplantation; PEI: Percutaneous ethanol injection; RF: Radiofrequency; PST: Performance status.
Multidisciplinary management of HCC
Given the complexity of the clinical scenario, the decision on the most appropriate treatment for a patient with HCC should be made by a multidisciplinary team that includes a hepatologist, hepatobiliary surgeon, transplant surgeon, radiologist and pathologist[18]. No single treatment strategy can be applied to all patients, and treatment should be individualized.
In the management of HCC, attention must be focused on the presence and degree of the underlying chronic liver disease at first observation, which will influence the choice of the treatment[19].
Liver resection can be offered to patients with well-preserved liver function; however, the amount of parenchyma that can be removed in carriers of chronic liver disease is inferior to that which is considered as the safety limit in a normal liver (a “future remnant liver” that is ≥ 30% of the hepatic volume is generally considered acceptable)[19]. Despite a normal liver function, the regenerative potential of a liver that harbors a chronic disease can be surprisingly low.
In cases of impaired liver function, non-surgical procedures or liver transplantation (LT) can be offered; in this setting, the number, size and location of the nodules determine the choice of treatment.
Surgical resection, transplantation and ablation are the treatments that offer the highest rates of complete response and are therefore considered as curative[10]. There are no randomized trials comparing the efficacy of these three approaches, and all evidence is based on the rate of cure reported in different series.
Transarterial chemoembolization (TACE) is the least invasive approach; however, it cannot be considered curative.
Ideally, LT is best treatment for early stage HCC on cirrhosis because it removes both the tumor and the chronic disease that has produced it. However, the application of this powerful tool is limited by the scarcity of donors, which results in strict patient selection criteria to optimize the results.
Candidates for LT for whom a long waiting time (> 6 mo) is predicted may be offered resection, local ablation or transarterial chemoembolization as a ‘bridge’ to transplantation to minimize the risk of tumor progression while they are on the waiting list[20]. The same procedures can also be utilized in attempts downstage tumors that are beyond the eligibility criteria for LT at the time of diagnosis.
Yu et al[21] performed a study on HCC patients who exceeded the University of California San Francisco (UCSF) criteria for LT; these patients were downstaged to fit the UCSF criteria using locoregional therapy and finally underwent LT. Patients who were successfully downstaged prior to transplantation had tumor-free and overall survival rates similar to those observed in patients who met the criteria from the beginning.
A 5-year survival comparable to that of “within criteria” HCC patients can be achieved using LT after successful downstaging. Successful downstaging should include tumor size, number of viable tumors and s-αFP concentrations before and after downstaging. Then, a minimum observation period of 3 mo is recommended before considering LT[22].
If a patient’s hepatic function allows it, liver resection can be offered prior to future transplantation by pursuing two different strategies: first, resection can be used as the primary therapy with LT offered as a rescue therapy should the patient develop tumor recurrence or postoperative liver failure (salvage transplantation); second, resection can be performed on patients with a high risk of tumor progression while awaiting transplantation (bridge to transplantation)[23,24].
SURGICAL RESECTION
When performed in specialized centers, hepatic resection (HR) can be highly effective, with 5-year overall survival rates well above 50% in the major series[25]. Resection is the recommended treatment for patients without advanced fibrosis as long as an R0 resection can be performed with a low risk of postoperative liver failure[14].
The elements that must be taken into account when considering resection of the cirrhotic liver are the Child-Pugh and MELD scores of the underlying liver disease, the degree of portal hypertension and the extension of the parenchymal excision required to obtain a free resection margin.
The aim of HR is to obtain radical resection with limited surgical morbidity; to achieve this goal, patient selection is crucial. For the last several decades, the selection of candidates for resection has been based on Child-Pugh classification. However, Child-Pugh classification is far from accurate for predicting postoperative liver failure; in fact, some Child-Pugh A patients already have liver functional impairment with an increased bilirubin concentration, clinically significant portal hypertension or even minor fluid retention necessitating diuretic treatment[26]. The further investigation of hepatic functional reserve tests, such as the aminopyrine breath test or clearance of indocyanine green (ICG), has been proposed; however, their predictive value remains poorly validated. In Japan, the ICG retention rate is utilized to identify the best candidates for resection[27], whereas portal pressure and bilirubin are the variables used in Europe and the United States[10]. Recently, a preoperative MELD score ≥ 10 was associated with a higher incidence (40%) of postoperative liver failure[28].
Markers of portal hypertension including a porto-caval gradient > 10 mmHg, the presence of esophageal varices, splenomegaly and a platelet count lower than 1× 1011/L are predictors of postoperative morbidity and mortality[29]. In patients without relevant portal hypertension and normal concentrations of bilirubin, the 5-year survival is 70%, whereas this value is 50% for individuals with portal hypertension and is even lower when both these risk factors are present[30,31].
By integrating all of these factors, HR can be safely performed on patients with Child-Pugh class A chronic liver disease, a MELD score ≤ 10, a platelet count > 100.000/mm3 and a porto-caval gradient <10 mmHg. These factors dramatically limit the potential number of candidates, and overall, less than 30% of patients are candidates for HR[29].
After HR, the 5-year survival for cirrhotic patients with HCC ranges from 30% to 50%, whereas the operative mortality ranges from 3% to 8%[29]. The severity of cirrhosis, size of the tumor, number of tumors, presence of vascular tumor invasion and presence of satellite nodules are well-established prognostic factors for recurrence and survival[27,32,33]. Late recurrence is mainly due to the carcinogenic effect of underlying chronic liver disease[34].
Absolute contraindications to HR are the presence of extrahepatic metastases or neoplastic invasion of the main portal trunk. Neoplastic portal vein thrombosis is a poor prognostic factor; however, in highly selected cases, hemi-hepatectomy can be feasible, particularly when thrombosis of a main branch of the portal vein has led to hypertrophy of the contralateral hemiliver.
When compared with open surgery, laparoscopy in cirrhotic patients could have the advantage of avoiding the interruption of collateral abdominal veins that are present as a result of portal hypertension. Several studies have indeed demonstrated the benefits of the laparoscopic approach in terms reduced bleeding and lower postoperative morbidity and mortality[35,36].
LIVER TRANSPLANTATION
HCC is the only solid cancer that can be treated with transplantation. Transplantation is the best curative option for patients with decompensated (Child-Pugh B or C) cirrhosis; however, due to the shortage of donors, it can only be offered to a limited number of patients. Candidates for LT are patients with tumors that have favorable pathological features and therefore a low likelihood of recurrence.
The most widely adopted criteria for selecting HCC carriers for transplantation are the Milan criteria. According to these criteria, LT can be considered only for patients with a single tumor < 5 cm in diameter or for patients with up to 3 tumors < 3 cm without macrovascular invasion[17]. A recent systematic review of 90 studies that followed 17780 patients over a 15-year period identified the Milan criteria as an independent prognostic factor of outcome after LT[37].
Despite recent discussions concerning the restrictive nature of the Milan criteria, these criteria have been adopted by the vast majority of transplant centers. The results of LT in HCC within the Milan criteria are outstanding, with 5-year survival approaching 80%, which is similar to that observed in patients transplanted for benign diseases[30,38,39]. Outside the Milan criteria, survival is significantly reduced, which is likely due to an increased prevalence of variables associated with risk of recurrence, such as microvascular invasion, in tumors at more advanced stages[40].
Neoadjuvant therapy through TACE can occasionally downstage tumors that were outside the Milan criteria at the time of diagnosis; in these cases, the results of LT are similar to those achieved when the criteria were met at first observation[41].
The time spent on the waiting list is a key factor that must be considered when assessing the results of LT in HCC patients; it depends on the availability of donors in a given area and on the system used to prioritize organ allocation. It was demonstrated that 15%-20% of patients with HCC initially within the Milan criteria experience tumor progression until they dropped out from the waiting list; this highlights the need to analyze the results of LT using an intention to treat approach[42]. Although treatments aimed at delaying tumor progression, such as ablation and transarterial chemoembolization, are widely used, their efficacy is unproven[43]. Live donation is a valid strategy for extending the donor pool; however, its applicability is reduced because of societal constraints, scarcity of appropriate donors and the possible morbidity and mortality of donors[44].
Currently, organs are allocated worldwide based on MELD score; however, HCC patients with low MELD scores are given extra points to shorten their waiting time and avoid tumor progression. This policy is questioned by some authors because it might be disadvantageous for patients without HCC[45].
LOCOREGIONAL TREATMENTS
Local ablation techniques have been developed for patients with surgical contraindications and can be performed either through a percutaneous approach or, less commonly, through laparoscopy. These maneuvers include percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), microwave ablation, cryoablation, laser-induced thermotherapy, high-intensity focused ultrasound and irreversible electroporation[46].
The first percutaneous treatment was PEI, which induces coagulative necrosis of the lesion as a result of cellular dehydration, protein denaturation and chemical occlusion of small tumor vessels due to the effects of the injected absolute alcohol. Ablation techniques such as RFA, microwave ablation, and laser ablation utilize high temperatures; conversely, cryoablation causes direct tumor freezing[47].
The evaluation of responses to locoregional treatments and molecular-targeted therapies of HCC is currently based on modified RECIST (mRECIST) criteria, which measure the diameter of the viable tumor component of target lesions[48].
Radiofrequency ablation
RFA is the technique of choice for local destruction of liver tumors. RFA induces coagulative necrosis of the tumor with safety margins around the lesion and is the most commonly used local ablative technique. RFA has largely replaced PEI because it produces better results in terms of recurrence-free survival and requires fewer treatment sessions[49]. RFA can be performed percutaneously under imaging guidance (ultrasound, CT or MRI) or during surgery guided by intraoperative US. The advantage of RF in the treatment of HCC in cirrhotic patients is that it allows selective destruction of the tumor, sparing the surrounding parenchyma, and can be easily repeated in case of recurrence. Complete ablation of lesions smaller than 2 cm is possible in more than 90% of cases[50].
There are several major limits to the use of RF: (1) complete necrosis is rarely observed when the tumor diameter is > 3 cm or when the tumor is adjacent to a major blood vessel due to the cooling effect of the blood flow; (2) it is difficult to reach some areas of the liver parenchyma percutaneously (e.g., segment 1); (3) subcapsular lesions can undergo rupture in the peritoneum; (4) bladder injury can occur when lesions in segments IVb-5 are treated; and (5) targeting the lesion can be difficult under ultrasound guidance in livers with multinodular cirrhosis.
Taking these limitations into account, the benefit of RF in the treatment of HCC has been well demonstrated, with overall 5-year survival rates between 33% and 55% in selected series[51]. The effectiveness of RFA has led to the proposal of this technique as an alternative to HR.
In the only randomized prospective trial with balanced groups of patients comparing HR to RFA for HCC < 3 cm in patients with cirrhosis, no difference was observed in terms of overall and disease-free survival, whereas RF was associated with lower perioperative morbidity (4.2% vs 55.5%, P < 0.05) and mortality (0% vs 1.1%)[52]. However, in a retrospective comparative study of Child-Pugh class A patients, surgery was significantly more effective for patients with single tumors > 3 cm in diameter, with an overall 3-year survival of 66% after surgery (vs 37% after RFA, P = 0.004) and a 3-year disease-free survival of 44% (vs19%, P = 0.001)[53]. This observation was confirmed by subsequent studies performed by different groups[54-56]. A recent systematic review and meta-analysis of 12 controlled trials showed a not notable difference in the short-term effectiveness of RFA and HR in the treatment of early-stage hepatocellular carcinoma meeting Milan criteria, but the long-term efficacy of HR was better than that of RFA[57]. However, HR was associated with more complications and a longer hospital stay.
Rather than competing techniques, RFA and HR are effective therapeutic options that can be chosen based on the severity of chronic liver disease as well as the size and location of the tumor.
Transarterial chemoembolization
Transarterial chemoembolization (TACE) is the most commonly used initial treatment for unresectable HCC[58] and is also the first-line therapy for downstaging tumors that exceed the criteria for transplantation or to avoid tumor progression in patients awaiting LT. TACE may also be considered as a neoadjuvant treatment that can be utilized before HR or RF ablation to reduce tumor volume and possibly target satellite micrometastases[59].
The rationale behind TACE use is the well-characterized angiogenic activity of HCC that results in hypervascular arterial feeding. This technique depends on the intra-arterial infusion of a cytotoxic chemotherapeutic agent emulsioned with Lipiodol followed by embolization of the feeding vessels through a trans-arterial catheter[60]. TACE is a well-established treatment for HCC in cirrhotic patients, and its efficacy for improving survival compared with the other supporting treatments has been demonstrated[61].
The maximum and sustained retention of the chemotherapeutic agent is used as a measure of the success of TACE; thus, embolic microspheres are employed that have the ability to sequester chemotherapeutic agents. The concentration of these microspheres is increased within the tumor, and their contents are subsequently released in a controlled manner over a 1-wk period, which reduces the systemic toxicity to a minimum[62].
Doxorubicin-eluting beads (DEB) are another transarterial liver-directed therapy. The use of an eluting bead can be considered an improvement over conventional TACE. DEB are preformed, deformable microspheres that are loaded with doxorubicin (up to 150 mg per treatment). The pharmacokinetic profile of DEB significantly differs from that of conventional TACE; in particular, the peak drug concentration in the serum is lower for DEB-TACE compared with conventional TACE. An objective response rate of 70% to 80% according to the EASL criteria has been achieved[63]. One- and 3-year survival rates of 89.9% and 66.3%, respectively, have been reported in a heterogeneous cohort of patients with BCLC (stages A to C) treated with DEB-TACE[64].
Absolute contraindications for TACE are decompensated cirrhosis (Child-Pugh B ≥ 8, including jaundice, clinical encephalopathy and refractory ascites), extensive tumor with massive replacement of both lobes in their entirety, severely reduced portal vein flow (portal vein occlusion or hepatofugal blood flow), and a creatinine clearance < 30 mL/min[65].
Microwave ablation
Microwave ablation (MWA) is a potentially curative ablation procedure that has been proven to be safe in both percutaneous and intraoperative settings[66]. MWA can be utilized in patients with advanced liver disease and HCC, provides a more predictable ablation compared with RFA and requires fewer treatment sessions[21,67,68].
Microwave ablation creates an electromagnetic field in the tissues surrounding the ablation antenna with an extension of several centimeter, without flow of electrical current. Tissue within the MWA field heats rapidly to temperatures over 100 °C without the detrimental effects of tissue impedance, allowing a more rapid and consistent ablation[10]. Microwave ablation carries the risk of more severe injury to adjacent structures due to differences in energy delivery when compared with RFA; therefore, the operative approach is preferred over the percutaneous approach because it allows liver mobilization and protection of adjacent organs[66,69,70].
Radioembolization
Radioembolization is a newer hepatic transarterial technique that employs radioactive substances such as Iodine-131-labeled Lipiodol[71] or microspheres containing Yttrium-90[72]. This technique has been shown to be feasible and safe for the treatment of HCC in cirrhotic patients[73,74]. Microspheres are delivered to the tumor area for selective production of high energy and low penetration radiation. Radioembolization can be safely performed in patients with portal vein thrombosis due to the minimally embolic effect of 90Y microspheres[75]. The reported rate of complete tumor necrosis is 90% for patients with HCC < 3 cm[76], whereas the rate of complete necrosis after TACE varies widely in the literature, from 15% to 70%[77].
Radiation therapy
Radiation therapy is generally not considered an option in HCC consensus documents or national guidelines, primarily because of the lack of level 1 evidence[78]. However, experience with conformal radiation therapy (RT), intensity modulated RT, stereotactic body RT and particle therapy is rapidly increasing. RT should be considered as a treatment option in patients unsuitable for other established local therapies[78]. RT has also been used as a bridge to liver transplant and can be safely combined with locoregional therapies such as TACE[78,79].
SYSTEMIC TREATMENTS
Systemic treatment of HCC in cirrhotic patients is not effective due to the poor chemosensitivity of the tumor, and the impairment of hepatic function significantly increases the toxicity of chemotherapy.
Because the demonstrated benefits of systemic chemotherapy and hormonal treatments are lacking[80,81], molecular targeted therapies have recently been developed. Sorafenib, an inhibitor of multi-kinase, has antiproliferative and antiangiogenic activity, delays tumor progression and is currently the only agent with proven efficacy for the treatment of patients with advanced HCC[11,82-84]. This multitargeted tyrosine kinase inhibitor is a small molecule that inhibits vascular endothelial growth factor receptor, platelet-derived growth factor receptor, B-Raf, Fms-related tyrosine kinase and c-kit[85,86].
The use of sorafenib is currently recommended for patients with preserved liver function and advanced HCC who are not suitable for HR or LT and have failed to respond to locoregional treatments[87]. It is recommended that the treatment be continued until progression of the tumor is demonstrated. The main side effects associated with the use of sorafenib are diarrhea and hand-foot skin reaction; other possible side effects include anorexia, nausea, vomiting, weight loss, hoarseness of voice, asthenia and hypertension[52,88]. These effects can occasionally require dose reduction or treatment discontinuation. The potential benefit of sorafenib as adjuvant treatment after LT has not been demonstrated.
On the basis of a large randomized phase III study, the Sorafenib HCC Assessment Randomized Protocol[83], Sorafenib has been approved by the United States Food and Drug Administration for the treatment of patients with advanced HCC.
Studies are ongoing that aim to identify the best responders to Sorafenib; c-Jun N-terminal kinase (JNK) activity was positively correlated with the CD133 expression level and inversely correlated with the therapeutic response to Sorafenib. Accordingly, JNK activity may be considered as a new predictive biomarker for response to Sorafenib treatment[89].
To date, there is no second-line treatment for patients who are intolerant to Sorafenib or experience tumor progression while undergoing treatment.
COMBINED TREATMENTS
A combination of the different therapeutic approaches mentioned thus far is often required to address the different clinical peculiarities of HCC patients. For example, chemoembolization or RFA can be performed before radical surgery with curative intent (HR or LT) to allow effective tumor downstaging or reduce tumor growth. Different authors have proposed HR as a first-line therapy in patients who are candidates for LT[90]; however, the possible effect of previous surgery on the technical complexity of the liver transplantation procedure is a matter of debate[90,91]. One argument in favor of hepatic resection prior to LT is that histological analysis of the tumor can provide useful information regarding its oncological behavior: features such as the presence of a tumor capsule, the degree of differentiation or the presence of micro-vascular invasion can be precisely assessed in the surgical specimen and are well-known prognostic factors. However, there is no consensus among different authors regarding the influence that histological parameters should have on the therapeutic strategy; some authors suggest that LT should be contraindicated for patients with tumors that display poor prognostic histological criteria, whereas others recommend transplant priority in the presence of the same risk factors[23,92].
CONCLUSION
The treatment of HCC in cirrhotic patients has changed significantly over the past several decades and has become a major clinical issue. Patients with HCC on cirrhosis can benefit from several effective treatments that will improve their survival; however, the choice of the most appropriate options depends on several factors, namely: (1) severity of the underlying chronic liver disease; (2) stage of the tumor assessed viaimaging; (3) histological features of the tumor (when available); (4) availability of an active transplant program; (5) availability of a hepatobiliary surgical unit; and (6) availability of an experienced interventional radiology service.
Some of these variables may account for the different attitudes that are often observed. To ensure that the most effective treatment can be offered for a given case, a multidisciplinary approach is warranted, and professionals skilled in the administration of the different treatment types should be available. The increase in the incidence of HCC justifies the development of services related to the management of this tumor.
Footnotes
P- Reviewer: Scherubl H S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
Source




