Additional Analyses Presented in European and Hepatitis C Genotype 4 Patient Subgroups Underscore Benefit of Simeprevir-based Treatment Regimens
April 12, 2014 11:24 AM Eastern Daylight Time
LONDON--(BUSINESS WIRE)--Janssen R&D Ireland (Janssen) today announced positive new data from the clinical development programme of simeprevir, including final data from cohort 2 of the Phase 2 COSMOS study of the protease inhibitor simeprevir administered once daily with Gilead Sciences Inc.’s nucleotide inhibitor sofosbuvir, with and without ribavirin (RBV), in adult patients chronically infected with genotype 1 hepatitis C virus (HCV). The final data, along with an additional analysis from COSMOS cohort 1 and new subgroup analyses of Phase 3 data in European and genotype 4 HCV patients, were presented at The International Liver Congress™ 2014 of the European Association for the Study of the Liver (EASL) in London.
“Following the recent positive opinion for simeprevir from the Committee for Medicinal Products for Human Use in the European Union, we look forward to bringing this regimen to patients in Europe in the near future.”
Final Phase 2 Data from the Interferon-free COSMOS Study
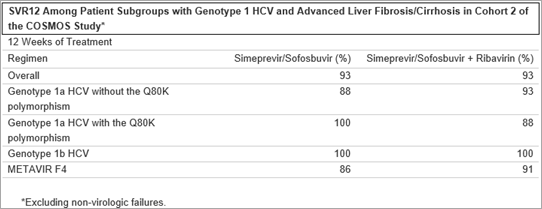
Cohort 21
Final results from cohort 2 of the Phase 2 COSMOS study* found that overall, 94 percent of genotype 1 treatment-naïve and prior null-responder HCV patients, with advanced liver fibrosis (METAVIR F3 or F4 scores) treated with simeprevir in combination with sofosbuvir, with or without RBV, for either 12 or 24 weeks achieved sustained virologic response 12 weeks after the end of treatment (SVR12). In patients treated with simeprevir and sofosbuvir alone, 93 percent and 100 percent of patients achieved SVR12 after 12 weeks and 24 weeks of treatment, respectively. The addition of RBV did not improve SVR rates; 93 percent of patients treated with the ribavirin-containing regimen achieved SVR12 after both 12 weeks and 24 weeks of treatment. Among HCV genotype 1a patients without Q80K, overall 97 percent of patients achieved SVR12 after 12 or 24 weeks of treatment regardless of treatment regimen, respectively. All patients with HCV genotype 1b achieved SVR12, regardless of treatment regimen or duration.
Among patients with baseline characteristics typically considered more difficult to treat, overall 98 percent of patients with the IL28B CT genotype, 95 percent of patients with the IL28B TT genotype, 95 percent of patients with METAVIR F4 scores, and 96 percent of genotype 1a patients with the Q80K polymorphism at baseline achieved SVR12. The most common adverse events reported during the study were fatigue, headache and nausea.
*Excluding non-virologic failures
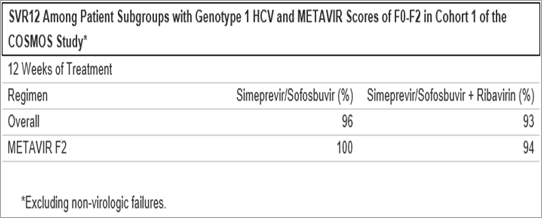
Cohort 12
An analysis of data from cohort 1 of the COSMOS study* demonstrated that overall 97 percent and 96 percent of genotype 1 HCV patients with METAVIR F0-F1 scores and F2 scores, respectively, treated with simeprevir and sofosbuvir alone achieved SVR12 after both 12 and 24 weeks of treatment. In patients treated with the ribavirin-containing regimen, 100 percent achieved SVR12 after 12 weeks and 24 weeks of treatment, respectively.
Among HCV genotype 1a patients with Q80K, 83 percent and 100 percent of patients treated with simeprevir and sofosbuvir alone achieved SVR12 after 12 or 24 weeks of treatment, respectively, compared to 89 percent of patients treated with simeprevir and sofosbuvir in combination with ribavirin. All patients with HCV genotype 1b achieved SVR12, regardless of treatment regimen or duration. Among patients with baseline characteristics typically considered more difficult to treat, overall 100 percent of patients with the IL28B CT genotype, 83 percent of patients with the IL28B TT genotype, and 100 percent of patients with IL28B CC genotype achieved SVR12. All patients without the Q80K polymorphism achieved SVR12, regardless of treatment regimen or duration. Adverse events (AEs) were mostly Grade 1/2 (77.5%); no serious AEs were reported, two patients discontinued treatment due to AEs.3
“The efficacy seen with the combination of simeprevir and sofosbuvir is very promising, especially considering the inclusion of patients with more advanced liver fibrosis in Cohort 2,” said Dr. Eric Lawitz, M.D., simeprevir clinical trial investigator, CEO at The Texas Liver Institute and Alamo Medical Research and Clinical Professor of Medicine at University of Texas Health Science Center. “I look forward to seeing the combination of simeprevir and sofosbuvir further evaluated in the recently initiated Phase 3 OPTIMIST trial.”
*Excluding non-virologic failures
Phase 3 Efficacy Data in European Patients with Genotype 4 Hepatitis C
Results from the Phase 3 RESTORE trial of simeprevir in combination with pegylated interferon and ribavirin in HCV genotype 4 treatment-naïve and treatment-experienced patients demonstrated that overall 65 percent of patients achieved SVR12, including 83 percent of treatment-naïve patients, 86 percent of prior relapsers, 60 percent of prior partial responders, and 40 percent of prior null responders. Among patients with genotype 4a and 4d HCV, 69 percent and 52 percent achieved SVR12, respectively.
In patients with the IL28B CT and TT genotypes, 66 percent and 60 percent achieved SVR12, respectively. Among patients with more severe liver fibrosis characterized by a METAVIR score of F3 or F4, 67 percent and 47 percent achieved SVR12, respectively. The most frequent adverse events included influenza-like illness, asthenia (weakness) and fatigue. Genotype 4 HCV is considered particularly difficult to treat and currently only limited treatment options are available.4
Subgroup Analyses of European Patients from Phase 3 Studies of Simeprevir
Analyses of pooled efficacy data from the QUEST-1 and QUEST-2 studies found 87 percent of European patients treated with simeprevir in combination with pegylated interferon (PegIFN) and RBV achieved SVR12, compared to 81 percent in the overall study population.5 In an analysis from the PROMISE study, 88 percent of European patients treated with simeprevir in combination with PegIFN and RBV achieved SVR12 compared to 79 percent in the overall study population.6
The efficacy of simeprevir in combination with pegylated interferon and ribavirin was also observed among European patients with baseline characteristics typically considered more difficult to treat. In QUEST-1 and QUEST-2, 71 percent of patients with METAVIR F4 scores, 86 percent of patients with the IL28B CT genotype, 69 percent of patients with the IL28B TT genotype and 64 percent of genotype 1a patients with the Q80K polymorphism at baseline achieved SVR12 in the simeprevir arm, compared to 25 percent, 44 percent, 31 percent and 50 percent of patients in the placebo arm, respectively. In PROMISE, 85 percent of patients with METAVIR F4 scores, 88 percent of patients with the IL28B CT genotype, 77 percent of patients with the IL28B TT genotype and 75 percent of genotype 1a patients with the Q80K polymorphism at baseline achieved SVR12 in the simeprevir arm, compared to 30 percent, 41 percent, 18 percent and 57 percent of patients treated in the placebo arm, respectively.5,6
“The data presented at EASL further reinforce the benefit of simeprevir-based treatment across diverse patient populations, including European patients,” said Gaston Picchio, Hepatitis Disease Area Leader, Janssen Research & Development. “Following the recent positive opinion for simeprevir from the Committee for Medicinal Products for Human Use in the European Union, we look forward to bringing this regimen to patients in Europe in the near future.”
About Simeprevir
Simeprevir is an NS3/4A protease inhibitor jointly developed by Janssen R&D Ireland and Medivir AB and indicated for the treatment of chronic hepatitis C infection in combination with pegylated interferon and ribavirin in genotype 1 HCV infected patients with compensated liver disease, including cirrhosis.
Janssen is responsible for the global clinical development of simeprevir and has exclusive, worldwide marketing rights, except in the Nordic countries. Medivir AB retains marketing rights for simeprevir in these countries under the marketing authorization held by Janssen-Cilag International NV. Simeprevir was approved for the treatment of genotype 1 hepatitis C in September 2013 in Japan, in November 2013 in Canada and the U.S., and in March 2014 in Russia. A Marketing Authorisation Application was submitted to the European Medicines Agency (EMA) in April 2013 by Janssen-Cilag International NV seeking approval of simeprevir for the treatment of genotype 1 or genotype 4 chronic hepatitis C and the Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion, recommending Marketing Authorisation in the European Union for the use of simeprevir in combination with other medicinal products for the treatment of chronic HCV. This application is under review by the EMA.
About Hepatitis C
Hepatitis C (HCV) is a major global public health concern. It is a serious and complex blood-borne virus which manifests itself through complications of the liver. If left untreated, it can cause significant and potentially fatal damage to the liver including cirrhosis, leading to eventual transplantation. In Europe it is a leading cause of liver transplantation.7
The World Health Organisation (WHO) and the European Association for the Study of the Liver (EASL) estimate that 150 million people worldwide were chronically infected with HCV in 2011.8 The virus is responsible for 350,000 deaths globally8 and 86,000 deaths in the European region each year.9 As the disease is often asymptomatic in its early stages it can be difficult to diagnose and treat. Up to 90 percent of those with HCV do not clear the virus without treatment and become chronically infected.10 The WHO estimates that 20 percent of people with HCV will develop cirrhosis and, of those, up to 20 percent may progress to liver cancer.11 Genotype 1 HCV is the most prevalent form of the virus worldwide12 and one of the most challenging to treat successfully.
About Janssen Pharmaceutical Companies
At Janssen, we are dedicated to addressing and solving some of the most important unmet medical needs of our time in oncology, immunology, neuroscience, infectious diseases and vaccines, and cardiovascular and metabolic diseases. Driven by our commitment to patients, we develop innovative products, services and healthcare solutions to help people throughout the world. Janssen R&D Ireland is part of the Janssen Pharmaceutical Companies of Johnson & Johnson. Please visit http://www.janssenrnd.com for more information.
References
__________________________
1 Lawitz M et al. The COSMOS cohort 2 study, abstract presented at the European Associate for the Study of the Liver (EASL) 2014.
2 Sulkowski MS et al. The COSMOS study, oral presentation presented at the European Association for the Study of the Liver (EASL) 2014.
3 Sulkowski MS et al. The COSMOS study, abstract presented at the European Associate for the Study of the Liver (EASL)
4 Moreno C et al. The RESTORE study, abstract presented at the European Association for the Study of the Liver (EASL) 2014.
5 Foster GR et al. The QUEST 1 and 2, abstract presented at the European Association for the Study of the Liver (EASL) 2014.
6 Forns X et al. The PROMISE study, abstract presented at the European Association for the Study of the Liver (EASL) 2014.
7 European Association for the Study of the Liver. EASL The Burden of Liver Disease in Europe. Available from http://www.easl.eu/assets/application/files/54ae845caec619f_file.pdf. Accessed March 2014.
8 World Health Organisation. Hepatitis C. Fact sheet N. 164. Available at: http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed March 2014.
9 Muhlberger M et al. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health 2009:9,34.
10 World Health Organisations (WHO). “Hepatitis C: About HCV Infection.” Available at: www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index3.html Accessed March 2014.
11 World Health Organisation. Hepatitis C. Available at: http://www.who.int/csr/disease/hepatitis/Hepc.pdf. Accessed March 2014
12 Zein NN. Clinical Significance of Hepatitis C Virus Genotypes. Clin. Microbiol. Rev. April 2000:13(2),223-235.
Contacts
Janssen EMEA
Media:
Hans Vanavermaete
Mobile: +32 (0) 478 447278
or
Rikki Jones
Mobile: +44 (0) 75 9591 9643
or
Investors:
Stan Panasewicz
Office: +1 (732) 524 2524
or
Louise Mehrotra
Office: +1 (732) 524 6491
Source
Also See: Final data from the phase II COSMOS study with Simeprevir in combination with Sofosbuvir presented at EASL (Medivirs’ Press Release)