Current Opinion in Gastroenterology
Mitchell L. Shiffman
Curr Opin Gastroenterol. 2014;30(3):217-222.
Abstract and Introduction
Abstract
Purpose of review: The evolution of treatment for patients with chronic hepatitis C virus (HCV) is evolving at a rapid pace. Two new oral antiviral agents, simeprevir and sofosbuvir, have already been approved and are now available for treatment of patients with chronic HCV. Other antiviral agents will be available during 2014.
Recent findings: The protease inhibitor simeprevir was recently approved for use with peginterferon (PEGINF) and ribavirin (RBV) in patients with chronic genotype 1. About 80% of patients achieve a rapid virologic response and can be treated for 24 weeks. The sustained virologic response (SVR) in treatment-naive patients is about 80%. Sofosbuvir, the first polymerase inhibitor, is effective in all HCV genotypes. When utilized with peginterferon and RBV for 12 weeks in treatment-naive patients with genotypes 1, 4, 5 and 6, an SVR of 90% is observed. Sofosbuvir and RBV have also been studied without interferon and represent the first interferon-free therapy for chronic HCV.
Summary: It is now possible to cure chronic HCV in the vast majority of patients with chronic HCV and in many patients without interferon.
Introduction
The treatment of chronic hepatitis C virus (HCV) continues to evolve at an accelerating pace. In 2011, the first two protease inhibitors, telaprevir and boceprevir, were approved to be utilized with peginterferon (PEGINF) and ribavirin (RBV) to treat chronic HCV genotype 1.[1–5] The addition of a protease inhibitor to PEGINF and RBV represented a huge advance in HCV treatment and increased sustained virologic response (SVR) in the treatment-naive population with HCV genotype 1 from about 40% to 70–75%. The main limitation of these first-generation protease inhibitors was side-effects, particularly anemia, which were more severe than observed with PEGINF and RBV. These adverse events are even more severe and increase the risk of hepatic decompensation in patients with cirrhosis. In a study that included only patients with advanced fibrosis or cirrhosis, many of whom had previously failed PEGINF and RBV, nearly half of all patients treated with either telaprevir or boceprevir developed serious adverse events, 25% discontinued treatment, over half developed severe anemia and required a hematopoetic growth factor and 1–2% died as a result of hepatic decompensation.[6] The patients at greatest risk to develop hepatic decompensation included those with thrombocytopenia and a low serum albumin.[7] The SVR in this cohort was only 40%. In the subset of patients with cirrhosis who failed previous therapy, the SVR was under 20%.[6,7] Results like these caused many physicians who were treating HCV to pause and wait for a better alternative.
In late 2013, another protease inhibitor simeprevir and the first polymerase inhibitor, sofosbuvir, were approved for HCV treatment. These two antiviral agents offer significant advantages compared with telaprevir and boceprevir when treating patients with HCV genotype 1; the duration of therapy is shorter, the adverse effect profile is superior and the SVR is higher. In addition, sofosbuvir is effective against all genotypes and when utilized with RBV represents the first interferon-free treatment for chronic HCV.
During the past few years, several oral antiviral agents, which inhibit various HCV proteins, have been developed at a rapid pace. These include protease inhibitors, nucleotide and nonnucleotide polymerase inhibitors, NS5A inhibitors and cyclophilin inhibitors. Two or more oral antiviral agents from different classes have been combined and their evaluation in phase 3 clinical trials is well underway.[8–11] During 2015 multiple oral antiviral combinations are expected to be available to treat chronic HCV (Table 1). The rapid evolution of these treatments will make any recommendations for how to treat HCV in 2014 tentative at best. The treatments that will be available during 2014 are illustrated in Fig. 1. The rapid evolution of HCV treatment has also occurred at a pace that far exceeds the appearance of peer-reviewed publications. The vast majority of cited references are therefore abstracts, which have been presented at national and international meetings during 2013.
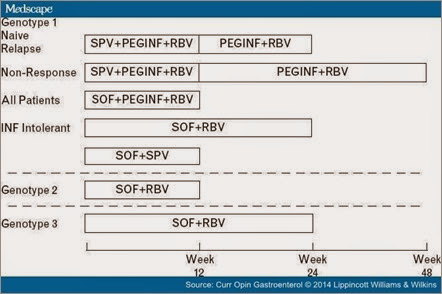
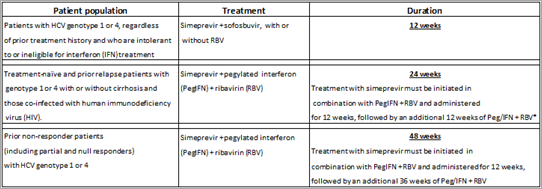
Figure 1.
Treatment regimens for patients with chronic hepatitis C virus (HCV) utilizing the two newest antiviral agents simeprevir (SPV) and sofosbuvir (SOF). Treatment of patients with HCV genotype 1 includes SPV, peginterferon (PEGINF) and ribavirin (RBV) for 24–48 weeks, SOF, PEGINF and RBV for 12 weeks, SOF plus RBV for 24 weeks or SOF and SPV for 12 weeks. The treatment of patients with HCV genotype 2 is SOF plus RBV for 12 weeks. The treatment of patients with HCV genotype 3 is SOF plus Simeprevir and Faldaprevir
Both simeprevir and faldaprevir are NS3–4A protease inhibitors.[12–16] Both act at the same binding site as telaprevir and boceprevir and are only effective in patients with HCV genotype 1. As a result, neither of these agents is likely to be effective in patients with resistance to telaprevir or boceprevir.
Simeprevir was approved for use in patients with HCV genotype 1 in late 2013 and faldaprevir is expected to be approved in early 2014. Both of these protease inhibitors will be utilized as triple therapy with PEGINF and RBV for 12 weeks followed by an additional 12–36 weeks of PEGINF and RBV. In patients who are treatment-naive or who have had prior relapse with PEGINF and RBV, the recommended total duration of therapy when utilizing simeprevir is 24 weeks, that is 12 weeks of simeprevir, PEGINF and RBV followed by an additional 12 weeks of PEGINF and RBV. Approximately, 80% of these patients will achieve a rapid virologic response (RVR) and be HCV RNA undetectable within 4 weeks of initiating treatment. The SVR in these patients is approximately 90%.[12,13] It is recommended that patients with HCV RNA more than 25 IU/ml at either weeks 4, 12 or 24 stop treatment. In patients with prior nonresponse to PEGINF and RBV, the total duration of therapy is 48 weeks, that is 12 weeks of simeprevir, PEGINF and RBV followed by an additional 36 weeks of PEGINF and RBV. The SVR rate in these patients is 53–65%.[14] It is recommended that patients with HCV RNA more than 25 IU/ml at either weeks 4, 12 or 24 also stop treatment. It is anticipated that the recommendations for faldaprevir will be quite similar.
Simeprevir and faldaprevir offer significant advantages over telaprevir and boceprevir. The most important of these is that neither of these agents cause additional anemia compared with PEGINF and RBV.[12–16] All of these agents are dosed as a single once daily tablet, no special diet is required during dosing and no significant drug-drug interactions have been observed. Simeprevir was not noted to have any adverse events with greater frequency than PEGINF and RBV.[12–14] Faldaprevir was noted to have a slightly higher incidence of rash.[15,16] However, the rash was graded as only mild or moderate in all cases and no grade 3 rashes were observed. Faldaprevir was also associated with a mild increase in total bilirubin without elevations in liver transaminases or alkaline phosphatase.
Controlled clinical trials comparing the various antiviral agents utilized for treatment of patients with HCV genotype 1 have not been conducted. As such, no direct comparison regarding the relative effectiveness of these agents can be made. Both simeprevir and faldaprevir triple therapies were evaluated against a placebo control with PEGINF and RBV. As a result, the improvement in SVR with the protease inhibitor over control could be compared for all of the available protease inhibitors.[1–5,12–16] Such a comparison suggests that RVR and SVR rates are somewhat higher in patients treated with simeprevir or faldaprevir compared with telaprevir and boceprevir. The high RVR rates observed with simeprevir and faldaprevir allow 80% of patients to be treated for only 24 weeks and lead to the higher SVR rates.
The success of treatment in patients treated with simeprevir or faldaprevir, like other protease inhibitors, is dependent upon an effective interferon response and this is modulated by interleukin-28B genotype. In treatment-naive patients, the SVR approaches 90% in patients with interleukin-28B genotype CC and declines in patients with the CT and TT genotypes.[12,13,15] In patients with prior nonresponse to PEGINF and RBV, the SVR rates during retreatment with simepreivr or faldaprevir triple therapy follow a similar trend of interferon responsiveness; higher rates of SVR with prior partial response and the lowest SVR rates in prior null responders.[14,16]
The primary limitation of simeprevir is that a mutation at the Q80K loci of HCV adversely impacts the antiviral efficacy of simeprevir and leads to a significant reduction in SVR.[12,13,17] This mutation is present in about 40% of patients with HCV genotype 1A. The Q80K mutation is only rarely seen in HCV genotype 1B. The Q80K mutation in HCV has the greatest impact and significantly lowers SVR in patients who are genetically less sensitive to interferon. In contrast, patients with interleukin-genotype CC, who are highly sensitive to interferon, have similar SVR rates even if the HCV Q80K mutation is present.[12,13] When treating patients with genotype 1A, it is therefore important that the patient is interleukin-28 genotype CC or that HCV does not contain the Q80K mutation. Testing the patient for their IL28B genotype and/or evaluating HCV for the presence of this mutation should be strongly considered if simeprevir is to be utilized. Patients with HCV genotype 1 and the Q80K mutation who are IL28B genotype CT or TT are best treated by an alternative antiviral agent.
Sofosbuvir
Sofosbuvir is the first polymerase inhibitor to be approved for the treatment of chronic HCV. It is a nucleotide analog, which inhibits the NS5B polymerase and is effective in all HCV genotypes. It is incorporated into the growing RNA sequence during replication and acts as a chain terminator. The appearance of resistance to sofosbuvir is extremely limited and when this does occur the viral species is unable to persist.
Sofosbuvir was studied as triple therapy with PEGINF and RBV for just 12 weeks in patients with genotypes 1, 4, 5 and 6[18**] This was a single arm study with no comparison with PEGINF and RBV because of the marked differences in the duration of treatment. Over 90% of patients treated with sofosbuvir triple therapy were HCV RNA undetectable within 2 weeks and virtually all patients achieved a RVR. The overall SVR rate was 90%: 89% in patients with genotype 1 and 96% in patients with genotype 4. In patients with cirrhosis, the SVR rate was 80%. All seven of the patients with HCV genotypes 5 and 6 achieved SVR. Sofosbuvir triple therapy has not been evaluated in patients who failed either PEGINF and RBV or triple therapy with a protease inhibitor.
The combination of sofosbuvir and RBV represents the first interferon-free regimen approved for use to treat patients with chronic HCV. This combination was initially studied and is approved for use in patients with HCV genotypes 2 and 3.[18**,19**] In patients with HCV genotype 2, sofosbuvir and RBV yielded superior SVR rates compared with PEGINF and RBV. In treatment-naive patients, 12 weeks of sofosbuvir and RBV achieved SVR rates of 91 and 98% in patients with and without cirrhosis respectively. In patients who had previously failed PEGINF and RBV SVR rates of 96 and 60% were observed with 12 weeks of treatment. Extending the duration of treatment from 12 to 16 weeks did increase the SVR in this subgroup of patients to 78%. The recommended duration of sofosbuvir and RBV for patients with HCV genotype 2 is 12 weeks.
In patients with genotype 3, treatment with sofosbuvir and RBV for 12 weeks yielded an SVR rate of only 61% in patients without cirrhosis and 34% in patients with cirrhosis.[18**,19**] These SVR rates are very similar to that observed with PEGINF and RBV. Extending the duration of sofosbuvir and RBV to 16 and 24 weeks increased the SVR rate in all patients with genotype 3 to about 62 and 84%, respectively.[19**,20,21] As a result, the recommended duration of sofosbuvir and RBV for patients with genotype 3 is 24 weeks.
Sofosbuvir and RBV were also studied in patients with genotypes 1, 2 and 3 who had co-infection with HIV.[22] The duration of treatment for patients with genotypes 1 and 3 was 24 and 12 weeks for patients with HCV genotype 2. SVR rates of 76, 92 and 88% were observed for patients with genotype 1, 2 and 3, respectively. This study led to the approval of sofosbuvir and RBV for the treatment of HCV in patients co-infected with HIV.
Sofosbuvir and RBV have also been studied without interferon in patients with HCV and liver cancer awaiting liver transplant and in patients with post-liver transplant recurrent HCV.[23,24] The duration of sofosbuvir and RBV in all of these studies was for 24 weeks. SVR rates of about 75% were achieved in each of these populations. These studies led to the recommendation that sofosbuvir and RBV be utilized in patients with HCV genotype 1 who were unable to receive PEGINF. The recommended duration of therapy in these patients was 24 weeks.
Sofosbuvir is a well tolerated antiviral agent with minimal side-effects. In a study in which sofosbuvir and RBV were compared with placebo in patients who could not take PEGINF and RBV, the only side-effects with increased frequency above placebo were anemia and pruritus, both of which were attributed to RBV.[19**]
Mixing and Matching Antiviral Agents
Both simeprevir and sofosbuvir are currently approved and available for treatment of chronic HCV. The combination of simeprevir and sofosbuvir with or without RBV for either 12 or 24 weeks was evaluated in about 160 patients with HCV genotype 1. Many of these patients had prior nonresponse to PEGINF and RBV and about half had advanced fibrosis or cirrhosis.[25] Over 93% of all patients achieved SVR. Treating for 24 weeks or using RBV was no more effective than 12 weeks of treatment with just simeprevir and sofosbuvir alone, without RBV. The SVR rate in patients with genotype 1B or genotype 1A without the Q80K mutation was 100%. Patients with genotype 1A and the Q80K mutation had an SVR rate of about 90%. Although the regulatory authorities did not specifically approve the combination of simeprevir and sofosbuvir for the treatment of patients with HCV, the approval by the US Food and Drug Administration states that simeprevir and sofosbuvir are 'indicated for the treatment of chronic HCV infection as part of a combination antiviral regimen'. This opens the door for the use of these agents along to treat patients with HCV genotype 1 and provides a cheaper, shorter and probably superior SVR than 24 weeks of sofosbuvir and RBV.
Identification of Patients With Hepatitis C Virus
An estimated 4 million persons in the United States and 300 million persons worldwide are infected with HCV.[1] In the United States, the vast majority were infected in the 1960–1980s through the transfusion of blood products and injection drug use. Many of these patients are asymptomatic and have evaded detection for many years. The need to identify these patients is why the Center for Disease Control and the US Preventive Services Task Force has recommended that all persons born between the years of 1945–1965 be screened for HCV.[26*,27*] If all persons in these birth cohort years were screened, it is estimated that 75% of all persons with HCV in the United States would be identified.
Conclusion
It is now possible to cure HCV in the vast majority of patients with chronic HCV. SVR rates of 80–90% can be routinely achieved in patients with all HCV genotypes in as little as 12–24 weeks. Of the antiviral agents currently available, sofosbuvir appears to be the easiest to manage, the most efficacious and the antiviral agent with the broadest of indications. Patients with HCV genotypes 1, 4, 5 and 6 can be treated with sofosbuvir, PEGINF and RBV for 12 weeks. SVR rates of 90% or better are achieved. Patients with genotype 1 who are unable to tolerate PEGINF and patients with HCV genotype 3 can be treated with sofosbuvir and RBV for 24 weeks. SVR rates in these patients range from 75 to 83%. Patients with genotype 2 can be treated with sofosbuvir and RBV for 12 weeks with an SVR exceeding 90%. Simeprevir offers an SVR of about 80%, but requires 24 weeks of PEGINF and RBV. Patients with HCV genotype 1A and the Q80K mutation have SVR rates that are significantly reduced. Perhaps the best use for simeprevir is with sofosbuvir for 12 weeks in patients with HCV genotype 1. Our ability to eradicate HCV is on the horizon. However, this cannot be achieved unless patients are recognized and this will require screening in those persons at greatest risk, which is now defined by the year of their birth.
References
1. Poordad F, McCone J Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–1206.
2. Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
3. Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1207–1217.
4. Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364:2417–2428; 8.
5. Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 2011; 365:1014–1024.
6. Fontaine H, Hezode C, Dorival C, et al. SVR12 rates and safety of triple therapy including telaprevir or boceprevir in 221 cirrhotic non responders treated in the French early access program (anrs co20-CUPIC). J Hepatol 2013; 58 (Suppl 1):S27.
7. Bourlie` re M, Wendt A, Fontaine H, et al. How to optimize HCV therapy in genotype 1 patients with cirrhosis. Liver Int 2013; 33 (Suppl 1):46–55.
8. Kowdley KV, Lawitz E, Poordad F, et al. Safety and efficacy of interferon-free regimens of ABT-450/r, ABT-267, ABT-333+/_ ribavirin in patients with chronic HCV GT1 infection: results from the AVIATOR study. J Hepatol 2013; 58 (Suppl; abstr 3).
9. Everson GT, Sims KD, Rodriguez-Torres M, et al. Interim analysis of an interferon (IFN)- and ribavirin (RBV)-free regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 in treatment-naive, hepatitis C virus genotype 1-infected patients. J Hepatol 2013; 58 (Suppl; abstr 1423).
10. Zeuzem S, Soriano V, Asselah T, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl j Med 2013; 369:630–639.
11. Gane E, Hyland R, Ding X, et al. ELECTRON: 100% Suppression of Viral Load through 4 Weeks' Posttreatment for Sofosbuvir + Ledipasvir (GS-5885) + RBV for 12 Weeks in Treatment-naive and –experienced Hepatitis C Virus GT 1 Patients. 20th Conference on Retroviruses and Opportunistic Infections. Atlanta, GA, 2013. Abstract 41LB.
12. Manns M, Marcellin P, Poordad FPF, et al. Simeprevir (TMC435) with pegylated interferon/ribavirin for the treatment of chronic HCV genotype-1 infection in treatment-naive patients: results from QUEST-2, a phase 3 trial. J Hepatol 2013; 58 (Suppl; abstr 1413).
13. Jacobson I, Dore GJ, Foster GR, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatmentnaive patients: results from QUEST-1 a phase III trial. J Hepatol 2013; 58 (Suppl 1):S574.
14. Zeuzem S, Berg T, Gane E, et al. TMC435 in HCV genotype 1 patients who have failed previous pegylated interferon/ribavirin treatment: Final SVR24 results of the ASPIRE trial. J Hepatol 37:56. (suppl abstract 2).
15. Sulkowski MS, Asselah T, Lalezari J, et al. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naive patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology 2013; 57:2143–2154.
16. Sulkowski MS, Bourlie` re M, Bronowicki JP, et al. Faldaprevir combined with peginterferon alfa-2a and ribavirin in chronic hepatitis C virus genotype-1 patients with prior nonresponse: SILEN-C2 trial. Hepatology 2013; 57: 2155–2163.
17. Palanisamy N, Danielsson A, Kokkula C, et al. Implications of baseline polymorphisms for potential resistance to NS3 protease inhibitors in Hepatitis C virus genotypes 1a, 2b and 3a. Antiviral Res 2013; 99:12–17.
18. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
**This is the first study to demonstrate the effectiveness of sofosbuvir for the treatment of patients with chronic HCV.
19. Jacobon IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotypes 2 or 3 in patients without treatment options. N Engl J Med 2013; 368:1867–1877.
**This is the first manuscript to demonstrate that an all oral interferon-free regimen can lead to SVR for patients with chronic HCV.
20. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir + ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE trial. Hepatology 2013; 58 (Suppl Abstract 1085).
21. Lawitz E, Poordad F, Brainard DM, et al. Sofosbuvir in combination with PegIFN and ribavirin for 12 weeks provides high SVR rates in HCV-infected genotype 2 or 3 treatment experienced patients with and without compensated cirrhosis: results from the LONESTAR-2 Study. Hepatology 2013; 58 (Suppl Abstract LB4).
22. Sulkowski MS, Rodriguez-Torres M, Lalezari JP, et al. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients co-infected With HIV (PHOTON-1). Hepatology 2013; 58 (Suppl Abstract 212).
23. Curry MP, Forns X, Chung RT, et al. Pretransplant sofosbuvir and ribavirin to prevent recurrence of HCV infection after liver transplantation. Hepatology 2013; 58 (Suppl); Abstract 213.
24. Charlton MR, Gane EJ, Manns MP, et al. Sofosbuvir and ribavirin for the treatment of established recurrent hepatitis C infection after liver transplantation: preliminary results of a prospective, multicenter study. Hepatology 2013; 58 (Suppl Abstract LB2).
25. Jacobson IM, Ghalib RH, Rodriguez-Torres M, et al. SVR results of a once daily regimen of simeprevir plus sofosbuvir with or without ribavirin in cirrhotic and noncirrhotic HCV genotype treatment naive and prior null-responder patients. The COSMOS study. Hepatology 2013; 58 (Suppl AASLD abstract LB3).
26. Moyer VA, and the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013; 159:349–357.
*This study demonstrates the importance of baby boomer cohort screening to detect persons infected with HCV.
27. Smith BD, Morgan RL, Beckett GA, et al. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med 2012; 157:817–822.
* This study demonstrates the importance of baby boomer cohort screening to detect persons infected with HCV.
Source