World J Diabetes. 2014 February 15; 5(1): 52-58.
Published online 2014 February 15. doi: 10.4239/wjd.v5.i1.52.
Copyright ©2014 Baishideng Publishing Group Co., Limited. All rights reserved.
Sandip K Bose and Ranjit Ray.
Sandip K Bose, Ranjit Ray, Department of Molecular Microbiology and Immunology, Saint Louis University, St. Louis, MO 63104, United States
Ranjit Ray, Division of Infectious Diseases, Allergy and Immunology, Edward A Doisy Research Center, St. Louis, MO 63104, United States
Ranjit Ray, Department of Internal Medicine, Saint Louis University, St. Louis, MO 63104, United States
Author contributions: Bose SK performed literature search and wrote the initial draft of the paper; Ray R edited the paper and made additional changes as needed.
Supported by The National Institutes of Health, NO. DK080812
Correspondence to: Ranjit Ray, PhD, Division of Infectious Diseases, Allergy and Immunology, Edward A Doisy Research Center, 1100 S. Grand Blvd., 8th Floor, St. Louis, MO 63104, United States. rayr@slu.edu
Telephone: +1-314- 9779034 Fax: +1-314-7713816
Received November 9, 2013; Revised December 20, 2013; Accepted January 13, 2014;
Abstract
Approximately 170 million people worldwide are chronically infected with hepatitis C virus (HCV). Chronic HCV infection is the leading cause for the development of liver fibrosis, cirrhosis, hepatocellular carcinoma (HCC) and is the primary cause for liver transplantation in the western world. Insulin resistance is one of the pathological features in patients with HCV infection and often leads to development of type II diabetes. Insulin resistance plays an important role in the development of various complications associated with HCV infection. Recent evidence indicates that HCV associated insulin resistance may result in hepatic fibrosis, steatosis, HCC and resistance to anti-viral treatment. Thus, HCV associated insulin resistance is a therapeutic target at any stage of HCV infection. HCV modulates normal cellular gene expression and interferes with the insulin signaling pathway. Various mechanisms have been proposed in regard to HCV mediated insulin resistance, involving up regulation of inflammatory cytokines, like tumor necrosis factor-α, phosphorylation of insulin-receptor substrate-1, Akt, up-regulation of gluconeogenic genes like glucose 6 phosphatase, phosphoenolpyruvate carboxykinase 2, and accumulation of lipid droplets. In this review, we summarize the available information on how HCV infection interferes with insulin signaling pathways resulting in insulin resistance.
Keywords: Hepatitis C virus, Insulin resistance, Insulin receptor substrate 1, Protein kinase B, mammalian target of rapamycin/S6K1, Suppressor of cytokine signaling 3, Glucose transporter-4, Lipid metabolism, Anti-viral therapy
Core tip: Insulin resistance is one of the pathological features in patients with hepatitis C virus (HCV) infection and often leads to development of type II diabetes. Recent evidence indicates that HCV associated insulin resistance may result in hepatic fibrosis, steatosis, hepatocellular carcinoma and resistance to anti-viral treatment. In this review, we summarize the available information on how HCV infection interferes with insulin signaling pathways.
INTRODUCTION
Hepatitis C virus (HCV) contains a positive sense single stranded RNA genome and belongs to the family Flaviviridae and genus Hepacivirus[1]. HCV genome, 9.6 kb in length, is composed of a 5’ non-translated region (NTR), a long open reading frame (ORF) encoding a polyprotein and a 3’ NTR. The ORF encodes a polyprotein of about 3000 amino acids that is translated via an internal ribosome entry site at the 5’ NTR. The polyprotein is then cleaved by both cellular and viral proteases into at least 10 different proteins[1]. These include three structural proteins namely, core and two envelope glycoproteins (E1 and E2). In addition, a protein called F or ARFP can be produced from a frame-shift of the core protein[2]. An ion channel protein p7 is formed by cleavage of E2[3]. Non structural proteins of HCV include NS2, NS3, NS4A, NS4B, NS5A, and NS5B.
The primary host cell for HCV is hepatocytes but replication may also occur in other cell types, such as peripheral blood mononuclear cells, as well as in B and T cell lines[4,5]. HCV is a major cause of acute and chronic liver disease worldwide. More than 170 million people are currently infected with HCV[6]. Currently HCV vaccine is not available. Acute infection is usually asymptomatic, making early diagnosis difficult. Approximately 70% of acutely infected individuals fail to clear the virus and become chronically infected[7]. Chronic HCV infection is the leading cause for the development of liver fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and is the primary cause for liver transplantation in the western world. The sustained antiviral response rate in treatment of chronic HCV infection with interferon (IFN)-α with ribavirin is limited (about 30%-40%)[8,9]. Boceprevir and telaprevir protease inhibitors, have been shown to exhibit significantly higher rates of sustained virologic response (SVR) against HCV genotype 1 (about 65%-75%) as compared with peginterferon-ribavirin alone[10,11]. However, use of these antiviral agents display higher incidence of adverse events, such as rash, gastrointestinal disorders, and anemia.
Insulin resistance plays an important role in the development of various complications associated with HCV infection. Recent evidence indicates that HCV associated insulin resistance may result in hepatic fibrosis, steatosis, HCC and resistance to anti-viral treatment[12]. Thus, HCV associated insulin resistance is a therapeutic target at any stage of HCV infection. HCV modulates normal cellular gene expression and interferes with the insulin signaling pathway. The aim of this review is to summarize the currently available information on how chronic HCV infection interferes with insulin signaling pathways resulting in insulin resistance.
GLUCOSE UPTAKE AND INSULIN RESISTANCE
Glucose is a key metabolite essential for the production of energy (mostly ATP) which is required by cells. There are several mechanisms underlying increased glucose production. These include production of free glucose by increased glycogenolysis in the liver, increased gluconeogenesis, activation of forkhead box transcription factor (FoxO1) and improper insulin-glucagon hormonal balance, which stimulates increased glucose production[13]. Several factors contribute to elevated gluconeogenesis in diabetes, namely (1) increased supply of glucogenic precursors to the liver (glycerol, amino acids, free fatty acids), (2) increased lipid content, (3) increased cytokines and adipokines, and (4) decreased insulin receptor (IR) signaling in hepatocytes[13]. Glucose uptake into cells is regulated by the action of specific hormones, namely insulin and glucagon. Insulin is a peptide hormone secreted by the β-cells of the pancreatic islets of langerhans and maintains normal blood glucose levels by facilitating cellular glucose uptake, regulating carbohydrate, lipid and protein metabolism and promoting cell division and growth through its mitogenic effects[14]. The ability of insulin to stimulate glucose uptake into tissues is central to the maintenance of whole-body glucose homeostasis[15]. Type II diabetes mellitus (T2DM), occurs when the production of insulin is not sufficient to overcome a difficulty the body has in properly using insulin. This difficulty is called insulin resistance, resulting in increased glucose levels. Both forms of diabetes can pose an increased risk of major lifelong complications. In the case of insulin resistance, this includes a fivefold increased risk of coronary vascular disease, diabetic retinopathy and neuropathy[16-19]. Fatty liver is relatively common in overweight and obese persons with T2DM and is an aspect of body composition related to severity of insulin resistance, dyslipidemia, and inflammatory markers[20].
Glucose transporter-4 (GLUT-4) was shown to be the major isoform responsible for enhanced glucose uptake into muscle and adipose tissues following the secretion of insulin into the bloodstream[21,22]. The process of glucose uptake by cells requires a series of events to take place in a timely manner. It involves the binding of insulin to the IR resulting in subsequent phosphorylation and activation of IR substrate 1 and 2 (IRS-1/IRS-2), central molecules of the insulin signaling cascade[23,24]. This in turn activates protein kinase B (AKT) by phosphorylation of Ser473 and Thr308 residues. Activated AKT causes the translocation of GLUT-4 from intracellular compartments to the cell surface where it is required for glucose uptake[25]. Any change in the signaling is likely to induce insulin resistance which is associated with a number of pathophysiological changes including glucose intolerance, obesity, dyslipidemia and hypertension. Insulin resistance is a physiological condition in which cells fail to respond to the normal actions of the hormone insulin. The body produces insulin, but the cells in the body become resistant to insulin and are unable to use it as effectively, resulting in an attenuated biological response, leading to hyperglycemia[26]. Accumulation of ectopic lipid metabolites, activation of the unfolded protein response pathway, and innate immune pathways have all been implicated in the pathogenesis of insulin resistance[27]. During the course of insulin resistance several inflammatory cytokines and lipid metabolites, like free fatty acids, interrupt with the normal insulin signaling and promote T2DM.
CHRONIC HCV INFECTION AND INSULIN RESISTANCE
Epidemiological studies suggest that patients with chronic HCV infection have a significantly increased prevalence of T2DM as compared to hepatitis B virus infected patients[28-30]. Both insulin resistance and diabetes can adversely affect the course of chronic hepatitis C (CHC), leading to enhanced steatohepatitis and liver fibrosis[30-32]. Insulin resistance, associated with type 2 diabetes, can promote fatty liver, and excessive hepatic accumulation of fat may promote insulin resistance and therefore contribute to the pathogenesis of the metabolic syndrome[33]. Insulin resistance is a critical component of type 2 diabetes mellitus pathogenesis. Several mechanisms are likely to be involved in the pathogenesis of HCV-related insulin resistance[34]. Several cellular lesions have been associated with insulin resistance, but the precise mechanism by which HCV induces insulin resistance remains elusive with numerous viewpoints and opinions[30].
Impairment of IRS-1 and IRS-2 expression has been observed in the liver of patients with chronic HCV infection, as well as in HCV core transgenic mice, and from in vitro cell culture system[35-38]. HCV mediates dysfunction of the insulin signaling pathways via several distinct mechanisms, such as upregulating the expression of suppressors of cytokine signaling 3 expression[35], down regulation of peroxisome proliferator-activated receptors gamma (PPARγ)[36], activation of mammalian target of rapamycin (mTOR)/S6K1 pathway[38], and increased tumor necrosis factor-α (TNF-α) secretion[39].
MODULATION OF IR SUBSTRATE BY HCV
HCV modulates insulin signaling and IRS-1 via multiple mechanisms which have been presented in Figure 1. Ser/Thr phosphorylation of IRS-1 inhibits its association with the IR, which in turn inhibits tyrosine phosphorylation of IRS-1, required for its activation, and promotes degradation. Upregulation of serine phosphorylation of IRS-1 is a key negative feedback mechanism under physiological conditions to prevent the action of insulin. In an insulin-resistant state, an imbalance occurs between positive IRS-1 Tyr-phosphorylation and negative Ser-phosphorylation of IRS-1[40]. HCV core protein expression in hepatocytes upregulates Ser312 phosphorylation status of IRS-1 and modulates downstream Akt activity by inhibiting Thr308 phosphorylation[37]. Ser312 and Ser1101 phosphorylation of IRS-1 inhibits its association with the IR and stimulates degradation. HCV core protein induces insulin resistance by increasing Ser312 and Ser 1101 phosphorylation, marking its for degradation via the activated mTOR/S6K1 pathway[38], and subsequently blocking Tyr- phosphorylation of IRS-1 and Thr308 phosphorylation of Akt for the inhibition of glucose uptake. Activation of mTOR signaling also plays a key role in modulating IRS-1 activity. HCV genotype 2a infection significantly downregulates the expression of TSC1/TSC2, which in turn results in activation of downstream mTOR and S6K1[38]. Phosphorylation of IRS-1 at Ser1101 via the mTOR-S6K1 pathway may release IRS-1 from intracellular complexes, thereby enabling its degradation[41]. HCV significantly increases Ser1101 phosphorylation of IRS-1, which enables its degradation[38].
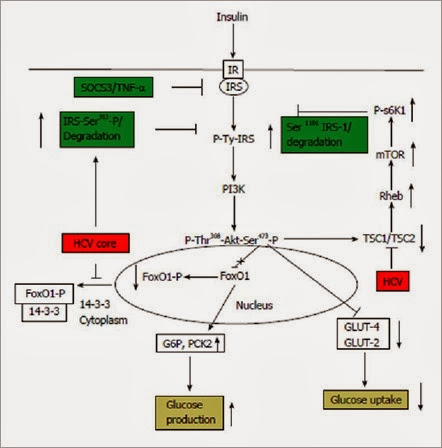
Figure 1 Schematic showing the interference of Hepatitis C virus in the insulin signaling pathway. Hepatitis C virus (HCV) core protein is known to up regulate Ser312 phosphorylation of insulin receptor substrate (IRS)-1 leading to degradation of IRS-1, the key molecule involved in propagation of insulin signal downstream from the insulin receptor (IR). HCV infection is also known to down regulate TSC1/TSC2 complex, resulting in subsequent upregulation of mTOR/S6K1 which leads to Ser1101 phosphorylation of IRS-1 and its subsequent degradation. A role of HCV mediated upregulation of SOCS3 and tumor necrosis factor-α (TNF-α) has also been proposed which leads to degradation and blocking of IRS-1 function. HCV also upregulates glucose 6 phosphatase (G6P), phosphoenolpyruvate carboxykinase 2 (PCK2) leading to increased glucose production, and down regulates glucose transporter (GLUT)-4, GLUT-2, leading to decreased glucose uptake by hepatocytes. Overall, these alterations lead to insulin resistance. mTOR: Mammalian target of rapamycin.
A decrease in expression of IRS-1 and IRS-2, in patients with HCV infection has also been reported[35]. Down-regulation of IRS-1 and IRS-2 was also seen in HCV core-transgenic mice livers and HCV core-transfected human hepatoma cells[35]. HCV core up-regulated suppressor of cytokine signaling 3 (SOCS3) and caused ubiquitination of IRS-1 and IRS-2. HCV core-induced down-regulation of IRS-1 and IRS-2 was not seen in SOCS3(-/-) mouse embryonic fibroblast cells, indicating the important role played by SOCS3 in mediating down regulation of IRS-1[35]. There have been reports that HCV genotypes might play an important role in deciding the pathway by which it impairs insulin signaling. It has been shown that the core protein of HCV genotype 3a promoted IRS-1 degradation through the downregulation of PPARγ and by upregulating the SOCS7, the core protein of genotype 1b activated the mTOR[36].
TNF-α, released in an excess may promote phosphorylation of serine residues of IRS-1 eventually leading to the downregulation of downstream insulin signaling molecule Akt. HCV core protein increases the expression level of TNF-α and promotes insulin resistance[42].
IMPAIRED LIPID AND GLUCOSE METABOLISM BY HCV
Insulin resistance is strongly influenced by abnormalities in lipid metabolism. Any dysfunction of the lipid metabolism triggers lipotoxicity through the production of free fatty acids thereby promoting insulin resistance[43]. HCV core protein down-regulates microsomal triglyceride transfer protein, an enzyme that mediates lipid translocation to the endoplasmic reticulum membrane and decreases the assembly of very low density lipoproteins[44]. It has been observed that HCV promotes fatty acid synthesis by the upregulation of lipogenic gene sterol regulatory element binding protein 1c which promotes the transcriptional activation of other lipogenic genes like acetyl CoA carboxylase, ATP citrate lyase, hydroxymethylglutaryl CoA reductase[45].
HCV infection promotes the expression of gluconeogenic genes namely, glucose 6 phosphatase (G6P) and phosphoenolpyruvate carboxykinase 2 (PCK2) resulting in increased glucose production and enhanced insulin resistance[46,38]. HCV also down regulates the expression of GLUT4, which is necessary for uptake of glucose. This results in a decreased glucose uptake and increased plasma glucose, leading to development of insulin resistance[38].
A schematic showing how HCV interferes with insulin signaling pathway, leading to insulin resistance is presented in (Figure 1). HCV modulates functioning of IRS-1 via multiple mechanisms, including up regulation of Ser312 or Ser1101 phosphorylation which leads to degradation of IRS-1. HCV also upregulates SOCS3 and down regulates TSC1/TSC2 leading to blocking of insulin signaling. HCV infection leads to increased gluconeogenesis via up regulation of G6P and PCK2. GLUT-4, and GLUT-2 expression is also down regulated by HCV leading to decreased glucose uptake. Overall, all these alterations by HCV leads to development of insulin resistance.
INSULIN RESISTANCE AND LIVER DISEASE PROGRESSION
The metabolic syndrome is a constellation of problems that includes insulin resistance, obesity, hypertension, and hyperlipidemia[47]. Increasingly, components of the metabolic syndrome are being linked to various forms of cancer, including the risk of developing HCC. IR is induced by HCV-4 irrespective of severity of liver disease. IR starts early in infection and facilitates progression of hepatic fibrosis and HCC development[47]. HCC patients showed higher IR frequency, and moderate to high viral load associated with high HOMA-IR in CHC and HCC[47]. Insulin resistance associates with a higher risk of HCC in cirrhotic HIV/HCV-co-infected patients also[48]. There are many causes of HCC, and nonalcoholic fatty liver disease (NASH) is emerging as a leading risk factor owing to the epidemic of obesity and T2DM. The mechanisms leading to HCC in obesity and T2DM likely involve interactions between several signaling pathways, many of which are modulated by HCV infection, and also include oxidative stress, inflammation, oncogenes, adiponectins, and insulin resistance associated with visceral adiposity and diabetes[49].
Insulin resistance and subsequent hyperinsulinemia are highly associated with fatty liver disease and is an important risk factor for the progression of fibrosis in CHC[50,51]. From metabolic aspect, HCV infection resembles NASH in numerous features, such as the presence of steatosis, serum dyslipidemia, and oxidative stress in the liver[52]. On the other hand, there are noticeable differences between hepatitis C and NASH, in the fact that HCV modulates cellular gene expression and intracellular signal transduction pathways, while such details have not been noted for NASH. HCV core protein expression leads to the development of progressive hepatic steatosis and HCC in transgenic mice[53]. Hepatic steatosis is known to occur at a high rate (40%-86%) in chronic HCV patients, and a close relationship between steatosis and intrahepatic core protein expression has been noted[54]. Insulin resistance is a prominent mechanism linking steatosis and fibrogenesis although this link is complex and not properly understood.
CLINICAL IMPLICATIONS OF HCV-MEDIATED INSULIN RESISTANCE
Several epidemiological, clinical and experimental data show that HCV plays a direct role in perturbing glucose metabolism, leading to both insulin resistance and diabetes[28-30]. Curing HCV results in the amelioration of insulin resistance and decreased incidence of diabetes after the end of therapy[55,56]. In the only trial that used the antidiabetic metformin[57], only a marginal, nonsignificant increase of the SVR rate was observed, despite an increased virological response after 4 wk of triple therapy. The data reported in a study using different schedules containing the antiglycaemic PPAR-γ agonist pioglitazone[58] are discouraging. Overall, the administration of insulin sensitizers together with the standard of care has not only failed to improve the virological response to therapy, but has also fallen short of providing much useful insight into the mechanisms linking reduced response to insulin resistance[59]. Early sulfonylureas although useful in lowering blood glucose level, were associated with significant off-target effects, and the biguanide phenformin was discontinued due to adverse events[60]. Although metformin is in the same drug class, it has a better safety profile and is now recommended as first-line treatment of diabetes during HCV infection.
THERAPEUTIC APPROACHES AND FUTURE GOALS
Treatment for HCV induced insulin resistance is highly linked with anti-viral treatment. Treatment of chronic HCV infection has 2 goals. The first is to achieve SVR (i.e., sustained eradication of HCV, which is defined as the persistent absence of HCV RNA in serum 6 mo or more after completing antiviral treatment). The second goal is to prevent progression to cirrhosis, HCC, and decompensated liver disease requiring liver transplantation. The treatment of HCV has evolved over the years. Current treatment options include combination therapy consisting of ribavirin and pegylated IFN. Protease inhibitors are emerging as a third feature of combination therapy. The sustained antiviral response rate in treatment of chronic HCV infection with IFN-α and ribavirin is limited (about 30%-40%)[8,9]. Boceprevir and telaprevir protease inhibitors have been shown to exhibit significantly higher rates of SVR against HCV genotype 1 (65%-75%) as compared with peginterferon-ribavirin alone[10,11]. More recently, sofosbuvir has also been used for treatment along with ribavirin, with significant increased SVR[61]. However, use of these antiviral agents display higher incidence of adverse events, such as rash, gastrointestinal disorders, and anemia. Thus, development of therapies with less side effects is desirable.
The prevalence of HCV antibodies in the type 2 diabetic population ranges between 1.78% and 12.1%[62]. Several cross-sectional studies have found a higher prevalence of HCV antibodies in type 2 diabetic patients than expected in the general population[62,63]. Early phase and total insulin secretion are determined using oral glucose tolerance testing (OGTT), Insulin sensitivity was measured directly by steady-state plasma glucose concentration during insulin suppression test. Fasting plasma glucose ≥ 126 mg/dL or 2-h plasma glucose > 200 mg/dL during OGTT are generally used as criteria for diagnosis of diabetes[64]. Well controlled DM was defined when the HbA1c level was < 7%. Agents used in diabetic therapy include the following: sulfonylureas, biguanides, alpha-glucosidase inhibitors, thiazolidinediones, Meglitinide derivativesetc[60]. Although effective in reducing blood glucose levels, early sulfonylureas were associated with significant off-target effects, and the biguanide phenformin was discontinued due to adverse events[60]. Although metformin is in the same drug class, it has a better safety profile and is now recommended as first-line treatment. However, many patients require additional glucose control treatment with an agent that has a complementary mechanism of action like metformin. Some common drugs used for treatment of T2DM available in the market include metformin oral, actos oral, Byetta subQ, Januvia oral, etc.
Another possible way of reversing insulin resistance would be via targeting the signaling components in the insulin signaling pathway modulated by HCV. For instance, we have shown that HCV up regulates phospho-S6K1, which stimulates degradation of IRS-1[38]. Thus, targeting phospho-S6K1 would be a target against HCV induced insulin resistance. These studies have not been done yet, so at this time it will be difficult to comment on the predictive outcome on reversal of insulin resistance. Use of specific inhibitors of SOCS-3, which may become useful to correct resistance to both insulin and IFN-α, are not available for clinical use. Alternatively, one may envision inhibiting TNF-α by administering infliximab or similar agents. IR also results from uncontrolled diet and life style. Regulation of weight, diet, and life style management will also be key in managing IR.
ACKNOWLEDGMENTS
We thank and Lin Cowick for preparation of the manuscript.
Footnotes
P- Reviewers: Efanov AM, Teeter JG, Traub M, Vestergaard ET S- Editor: Zhai HH L- Editor: A E- Editor: Liu SQ
References
1.Kato N. Genome of human hepatitis C virus (HCV): gene organization, sequence diversity, and variation. Microb Comp Genomics. 2000;5:129-151. [PubMed]
2.Walewski JL, Keller TR, Stump DD, Branch AD. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA. 2001;7:710-721. [PubMed]
3.Pavlović D, Neville DC, Argaud O, Blumberg B, Dwek RA, Fischer WB, Zitzmann N. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc Natl Acad Sci USA. 2003;100:6104-6108. [PubMed] [DOI]
4.Castillo I, Rodríguez-Iñigo E, Bartolomé J, de Lucas S, Ortíz-Movilla N, López-Alcorocho JM, Pardo M, Carreño V. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut. 2005;54:682-685. [PubMed] [DOI]
5.Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J. 2011;8:346. [PubMed] [DOI]
6.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35. [PubMed]
7.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [PubMed] [DOI]
8.Hoofnagle JH, di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356.[PubMed] [DOI]
9.Moradpour D, Blum HE. Current and evolving therapies for hepatitis C. Eur J Gastroenterol Hepatol. 1999;11:1199-1202. [PubMed]
10.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S; ADVANCE Study Team.Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] [DOI]
11.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI]
12.El-Zayadi AR, Anis M. Hepatitis C virus induced insulin resistance impairs response to anti viral therapy. World J Gastroenterol. 2012;18:212-224. [PubMed] [DOI]
13.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9-19. [PubMed] [DOI]
14.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19-39. [PubMed]
15.Leney SE, Tavaré JM. The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. J Endocrinol. 2009;203:1-18. [PubMed] [DOI]
16.
Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458. [PubMed] [DOI]
17.McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab. 2001;86:713-718. [PubMed] [DOI]
18.Abcouwer SF. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J Clin Cell Immunol. 2013;:(11).[PubMed] [DOI]
19.Hussain G, Rizvi SA, Singhal S, Zubair M, Ahmad J. Serum levels of TNF-α in peripheral neuropathy patients and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes Metab Syndr. 2013;7:238-242. [PubMed] [DOI]
20.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906-E916.[PubMed] [DOI]
21.Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305-315. [PubMed] [DOI]
22.Charron MJ, Brosius FC, Alper SL, Lodish HF. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci USA. 1989;86:2535-2539. [PubMed]
23.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182-186.[PubMed] [DOI]
24.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900-904. [PubMed] [DOI]
25.Olson AL, Knight JB. Regulation of GLUT4 expression in vivo and in vitro. Front Biosci. 2003;8:s401-s409.[PubMed]
26.Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp Biol Med (Maywood). 2001;226:13-26.[PubMed]
27.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852-871. [PubMed] [DOI]
28.Knobler H, Schattner A. TNF-{alpha}, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98:1-6.[PubMed] [DOI]
29.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [PubMed] [DOI]
30.Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World J Gastroenterol. 2010;16:1943-1952. [PubMed] [DOI]
31.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] [DOI]
32.Tazawa J, Maeda M, Nakagawa M, Ohbayashi H, Kusano F, Yamane M, Sakai Y, Suzuki K. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:710-715.[PubMed]
33.Weickert MO, Pfeiffer AF. Signalling mechanisms linking hepatic glucose and lipid metabolism. Diabetologia. 2006;49:1732-1741. [PubMed] [DOI]
34.Adinolfi LE, Durante-Mangoni E, Zampino R, Ruggiero G. Review article: hepatitis C virus-associated steatosis--pathogenic mechanisms and clinical implications. Aliment Pharmacol Ther. 2005;22 Suppl 2:52-55. [PubMed]
35.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [PubMed]
36.Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. [PubMed] [DOI]
37.Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606-2612. [PubMed] [DOI]
38.Bose SK, Shrivastava S, Meyer K, Ray RB, Ray R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J Virol. 2012;86:6315-6322. [PubMed] [DOI]
39.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [PubMed]
40.Virkamäki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931-943. [PubMed] [DOI]
41.Fritsche L, Weigert C, Häring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver--implications for health and disease. Curr Med Chem. 2008;15:1316-1329. [PubMed]
42.Pal S, Polyak SJ, Bano N, Qiu WC, Carithers RL, Shuhart M, Gretch DR, Das A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J Gastroenterol Hepatol. 2010;25:627-634. [PubMed] [DOI]
43.Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S28-S32. [PubMed]
44.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [PubMed] [DOI]
45.Kim KH, Hong SP, Kim K, Park MJ, Kim KJ, Cheong J. HCV core protein induces hepatic lipid accumulation by activating SREBP1 and PPARgamma. Biochem Biophys Res Commun. 2007;355:883-888. [PubMed] [DOI]
46.Deng L, Shoji I, Ogawa W, Kaneda S, Soga T, Jiang DP, Ide YH, Hotta H. Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J Virol. 2011;85:8556-8568.[PubMed] [DOI]
47.Mohamed AA, Loutfy SA, Craik JD, Hashem AG, Siam I. Chronic hepatitis c genotype-4 infection: role of insulin resistance in hepatocellular carcinoma. Virol J. 2011;8:496. [PubMed] [DOI]
48.Salmon D, Bani-Sadr F, Loko MA, Stitou H, Gervais A, Durant J, Rosenthal E, Quertainmont Y, Barange K, Vittecoq D. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: results from ANRS CO13 HEPAVIH. J Hepatol. 2012;56:862-868. [PubMed] [DOI]
49.Siddique A, Kowdley KV. Insulin resistance and other metabolic risk factors in the pathogenesis of hepatocellular carcinoma. Clin Liver Dis. 2011;15:281-96, vii-x. [PubMed] [DOI]
50.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [PubMed] [DOI]
51.Banerjee A, Meyer K, Mazumdar B, Ray RB, Ray R. Hepatitis C virus differentially modulates activation of forkhead transcription factors and insulin-induced metabolic gene expression. J Virol. 2010;84:5936-5946.[PubMed] [DOI]
52.Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179-187. [PubMed] [DOI]
53.Clément S, Pascarella S, Conzelmann S, Gonelle-Gispert C, Guilloux K, Negro F. The hepatitis C virus core protein indirectly induces alpha-smooth muscle actin expression in hepatic stellate cells via interleukin-8. J Hepatol. 2010;52:635-643. [PubMed] [DOI]
54.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] [DOI]
55.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. [PubMed] [DOI]
56.Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, Diago M, Alonso S, Planas R, Solá R, Pons JA, Salmerón J, Barcena R. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48:721-727. [PubMed] [DOI]
57.Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M, Moreno-Otero R, Nuñez O, Olveira A, Durán S, Planas R; Spanish Treatment of Resistance to Insulin in Hepatitis C Genotype 1 Group.Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702-1708. [PubMed] [DOI]
58.Overbeck K, Genné D, Golay A, Negro F; Swiss Association for the Study of the Liver (SASL).Pioglitazone in chronic hepatitis C not responding to pegylated interferon-alpha and ribavirin. J Hepatol. 2008;49:295-298.[PubMed] [DOI]
59.Negro F. Steatosis and insulin resistance in response to treatment of chronic hepatitis C. J Viral Hepat. 2012;19 Suppl 1:42-47. [PubMed] [DOI]
60.Guthrie RM. Evolving therapeutic options for type 2 diabetes mellitus: an overview. Postgrad Med. 2012;124:82-89. [PubMed] [DOI]
61.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804-811. [PubMed] [DOI]
62.Ozyilkan E, Erbaş T, Simşek H, Telatar F, Kayhan B, Telatar H. Increased prevalence of hepatitis C virus antibodies in patients with diabetes mellitus. J Intern Med. 1994;235:283-284. [PubMed]
63.Simó R, Hernández C, Genescà J, Jardí R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care. 1996;19:998-1000. [PubMed]
64.Mukhtar NA, Ayala C, Maher JJ, Khalili M. Assessment of factors associated with pre-diabetes in HCV infection including direct and dynamic measurements of insulin action. J Viral Hepat. 2012;19:480-487.[PubMed] [DOI]
Source

