Provided by NATAP
Download the PDF here
Download the PDF here
(from jules: this was an initial small study with only GS7977+Rbv - 83% African-American/70-80% genotype 1a/23% advanced liver disease/96% undetectable in rbv wt-based group at the end of 24 weeks treatment with 7 relapsers after that)
JAMA August 28, 2013
Anuoluwapo Osinusi, MD, MPH1,2; Eric G. Meissner, MD, PhD1; Yu-Jin Lee1; Dimitra Bon, MS3; Laura Heytens, RN4; Amy Nelson, RN1; Michael Sneller, MD1; Anita Kohli, MD1; Lisa Barrett, MD, PhD1; Michael Proschan, PhD5; Eva Herrmann, PhD3; Bhavana Shivakumar, MS1; Wenjuan Gu, PhD6; Richard Kwan, PAC4; Geb Teferi, MD7; Rohit Talwani, MD8; Rachel Silk, RN2; Colleen Kotb, RN2; Susan Wroblewski, RN1; Dawn Fishbein, MD9; Robin Dewar, PhD6; Helene Highbarger, MS6; Xiao Zhang, MS1; David Kleiner, MD10; Brad J. Wood, MD11; Jose Chavez, MD7; William T. Symonds, PharmD12; Mani Subramanian, MD, PhD12; John McHutchison, MD12; Michael A. Polis, MD, MPH1; Anthony S. Fauci, MD1; Henry Masur, MD4; Shyamasundaran Kottilil, MD, PhD1
Gilead HCV Program, GS-7977 - (08/19/13)
"Participants were enrolled in this single-center, 2-part, randomized controlled trial conducted at the Clinical Research Center of the National Institutes of Health, Bethesda, Maryland, from October 2011 through April 2012. Eligible participants were infected with HCV genotype 1, had liver biopsy-proven chronic disease, and were naive to HCV treatment..........Eighty-three percent of the participants were black; 66%, men; and 48%, body mass index greater than 30 (calculated as weight in kilograms divided by height in meters squared); 81% had the IL28 CT or TT genotype; 70%, GT-1a genotype; 23%, advanced liver disease; and 62%, baseline HCV RNA levels greater than 800 000...........
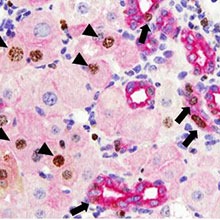
In the first part of the study, 9 participants (90%; 95% CI, 55%-100%) achieved SVR24. In the second part, 7 participants (28%) in the weight-based group and 10 (40%) in the low-dose group relapsed after treatment completion leading to SVR24 rates of 68% (95% CI, 46%-85%) in the weight-based group and 48% (95% CI, 28%-69%; P = .20) in the low-dose group. Twenty individuals participated in a pharmacokinetic-viral kinetic substudy, which demonstrated a slower loss rate of infectious virus in relapsers than in participants who achieved SVR (clearance, 3.57/d vs 5.60/d; P = .009). The most frequent adverse events were headache, anemia, fatigue, and nausea. There were 7 grade 3 events including anemia, neutropenia, nausea, hypophosphatemia, and cholelithiasis or pancreatitis. No one discontinued treatment due to adverse events....... Bivariable analysis of baseline factors showed that in all randomized participants who completed treatment, the odds of relapse was significantly higher in participants who were male (odds ratio [OR], 6.09; 95% CI, 1.17-31.6), had advanced fibrosis (OR, 4.27; 95% CI, 1.10-16.54), and baseline HCV RNA greater than 800 000 IU/mL (OR, 5.74; 95% CI, 1.35-24.38; Table 4). Given the small number of events and the exploratory nature of the stepwise analysis that determined variables used in the model, only the bivariable model results are reported herein........ Deep sequencing of all baseline samples showed no S282T resistant mutant......Twenty-nine participants (58%) had paired liver biopsies with an improvement in inflammation in 27 participants (93%) with a median drop of 5 points (15-point scale; eFigure 3A and B in the Supplement). In parallel with HCV RNA decline, there was rapid improvement of alanine aminotransferase levels with 77% normalizing by day 7 and 98% by day 14. A similar pattern was observed with aspartate aminotransferase levels (eFigure 4A in the Supplement"
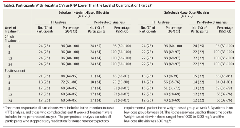
"........Twenty-four participants (96%) in each group achieved viral suppression by week 4. Four participants discontinued the study drug by week 8 due to nonadherence (1 in the weight-based group; 3 in the low-dose; Figure). One patient declined to continue study drug past week 12, but his viral load remained undetectable 24 weeks after stopping treatment. He is included in the final analysis. A total of 7 participants (28%) in the weight-based group and 10 (40%) in the low-dose group relapsed after treatment completion leading to SVR24 rates of 68% (95% CI, 46%-85%) in the weight-based group and 48% (95% CI, 28%-69%; P = .20) in the low-dose group (Table 2). A within-cohort comparison of baseline factors related to SVR was performed and is shown in eTable 3 in the Supplement. Deep sequencing of all baseline samples showed no S282T resistant mutant......."
"........All participants experienced a rapid decline in plasma HCV RNA. A viral kinetic model over the first 50 days of treatment of all randomized participants showed no differences in viral decay based on ribavirin dose or baseline characteristics (eFigure 1 and eFigure 2 in the Supplement). However, a fully fitted pharmacokinetics-viral kinetics model of a subset of 20 participants (10 in the low-dose group and 10 in the weight-based group) showed a significantly slower loss rate of free virus (clearance) in relapsers than participants who achieved SVR (clearance, 3.57 vs 5.60 per day; P = .009). There were no observable differences in viral decay, drug efficiency, loss rate of infected cells, or loss rate of infectious virus based on baseline characteristics (eTable 4 in the Supplement).......Twenty-nine participants (58%) had paired liver biopsies with an improvement in inflammation in 27 participants (93%) with a median drop of 5 points (15-point scale; eFigure 3A and B in the Supplement). In parallel with HCV RNA decline, there was rapid improvement of alanine aminotransferase levels with 77% normalizing by day 7 and 98% by day 14. A similar pattern was observed with aspartate aminotransferase levels (eFigure 4A in the Supplement)."
(Click table to enlarge)
ABSTRACT
Importance The efficacy of directly acting antiviral agents in interferon-free regimens for the treatment of chronic hepatitis C infections needs to be evaluated in different populations.
Objective To determine the efficacy and safety of sofosbuvir with weight-based or low-dose ribavirin among a population with unfavorable treatment characteristics.
Design, Setting, and Patients Single-center, randomized, 2-part, open-label phase 2 study involving 60 treatment-naive patients with hepatitis C virus (HCV) genotype 1 enrolled at the National Institutes of Health (October 2011-April 2012).
Interventions In the study's first part, 10 participants with early to moderate liver fibrosis were treated with 400 mg/d of sofosbuvir and weight-based ribavirin for 24 weeks. In the second part, 50 participants with all stages of liver fibrosis were randomized 1:1 to receive 400 mg of sofosbuvir with either weight-based or low-dose 600 mg/d of ribavirin for 24 weeks. Main Outcomes and Measures The primary study end point was the proportion of participants with undetectable HCV viral load 24 weeks after treatment completion (sustained virologic response of 24 weeks [SVR24]).
Results In the first part of the study, 9 participants (90%; 95% CI, 55%-100%) achieved SVR24. In the second part, 7 participants (28%) in the weight-based group and 10 (40%) in the low-dose group relapsed after treatment completion leading to SVR24 rates of 68% (95% CI, 46%-85%) in the weight-based group and 48% (95% CI, 28%-69%; P = .20) in the low-dose group. Twenty individuals participated in a pharmacokinetic-viral kinetic substudy, which demonstrated a slower loss rate of infectious virus in relapsers than in participants who achieved SVR (clearance, 3.57/d vs 5.60/d; P = .009). The most frequent adverse events were headache, anemia, fatigue, and nausea. There were 7 grade 3 events including anemia, neutropenia, nausea, hypophosphatemia, and cholelithiasis or pancreatitis. No one discontinued treatment due to adverse events.
Conclusion and Relevance In a population of patients with a high prevalence of unfavorable traditional predictors of treatment response, a 24-week regimen of sofosbuvir and weight-based or low-dose ribavirin resulted in SVR24 rates of 68% and 48%, respectively.
Chronic infection with hepatitis C virus (HCV) is a major cause of chronic liver disease, end-stage liver disease, hepatocellular cancer and remains the leading indication for liver transplants in western countries.1- 2 The HCV epidemic in the United States is centered in large urban areas among populations with a high prevalence of unfavorable traditional predictors of treatment response.1,3- 4 The addition of the recently approved directly acting antiviral agents telaprevir or boceprevir to pegylated interferon-alfa and ribavirin has resulted in improved sustained virologic response (SVR) rates; however, adverse reactions, high pill burdens, and drug interactions continue to make treatment challenging.5- 7 Furthermore, certain host and viral factors including black race, advanced liver fibrosis, IL28B CT or TT genotypes, high baseline HCV viral loads, and prior treatment experience appear to remain associated with poorer treatment outcomes.5,7- 9
Recent studies show that interferon-free, directly acting antiviral agent-only regimens can successfully achieve SVR; however, populations traditionally associated with poorer treatment outcomes have been underrepresented.10- 12 Many studies also use ribavirin, currently a standard component of interferon-based HCV therapy, which is associated with significant adverse events including hemolytic anemia, nausea, and teratogenicity.13 Although ribavirin clearly improves SVR rates with interferon-based therapies,14- 15 the role and requirement for ribavirin in emerging directly acting antiviral agent regimens, including optimal dosing, have not been established.
In this study, we evaluated the safety and efficacy of sofosbuvir administered in combination with weight-based or low-dose once daily ribavirin for 24 weeks in a treatment-naive population with unfavorable characteristics of treatment success. We report the efficacy of this regimen as defined by SVR rates 24 weeks after completion of treatment as well as the host and viral factors associated with treatment relapse.
METHODS
Participants
Participants were enrolled in this single-center, 2-part, randomized controlled trial conducted at the Clinical Research Center of the National Institutes of Health, Bethesda, Maryland, from October 2011 through April 2012. Eligible participants were infected with HCV genotype 1, had liver biopsy-proven chronic disease, and were naive to HCV treatment. Additional eligibility criteria included seronegativity for human immunodeficiency virus (HIV) and hepatitis B; absolute neutrophil count of 750 cells/μLor more; platelet count of 50 000 cells/μL or more; and hemoglobin of 11 g/dL or more for women and 12 g/dL or more for men. Race/ethnicity was classified as white, black, or Hispanic using patient self-reported data. Written consent was obtained from all participants except for 2 patients with limited literacy who gave oral consent after the entire constent form had been read and explained to them.
Study Design
The study was performed in 2 parts. In the first part (proof of concept), participants with early to moderate liver fibrosis (Knodell histology activity index [HAI] fibrosis score, 0-1) were treated for 24 weeks with 400 mg/d of sofosbuvir and weight-based ribavirin (400 mg in the morning, 600 mg in the evening if <75 kg or 600 mg twice a day if >75 kg). In the second part, eligible participants with all stages of fibrosis (including compensated cirrhosis) were randomized in a balanced fashion to receive 400 mg/d of sofosbuvir in combination with either weight-based ribavirin or low-dose (600 mg/d) of ribavirin for 24 weeks. The randomization used a set of 60 random numbers, in which blocks of 4 numbers were selected. Within a block, the highest numbers were assigned to the weight-based protocol, and the lower numbers were assigned to the low-dose group. Once enrollment occurred, participants received a study number in sequential fashion. Participants who experienced treatment failure were offered the current standard of care.
Study Oversight
The study was approved by the institutional review board of the National Institute of Allergy and Infectious Diseases (NIAID) and was conducted in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and regulatory requirements. An independent safety monitor participated in the interim safety and efficacy analysis.
Efficacy Assessments
Plasma HCV RNA levels were measured using the real-time HCV assay (Abbott Molecular), with a lower limit of quantification of 12 IU/mL and a lower limit of detection of 3 IU/mL. The Abbott assay was used to measure HCV RNA levels in all participants at all time points. Plasma HCV RNA levels were also measured using the COBAS TaqMan HCV RNA assay, version 1.0 (Roche), with a lower limit of quantification of 43 IU/mL and a lower limit of detection of 12 IU/mL at specified clinical time points.
Safety Assessments
Adverse events and clinical laboratory results were recorded throughout the study. Adverse events were graded from 1 (mild) to 4 (severe) by a standardized scale using the Division of AfIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (Division of AIDS toxicity table version 1.0). Patient adherence was determined by pill counts at each visit and during patient interviews. A missed dose was defined as any component of the medication regimen not taken on a given day.
Viral Kinetics, Pharmacokinetics, and Pharmacodynamics
Early viral kinetics, pharmacokinetics, and pharmacodynamics of sofosbuvir and its metabolite GS-331007 were obtained and calculated. Levels of sofosbuvir and its metabolite GS-331007 in serum were measured at 0, 1, 2, 4, 8, 12, 24, and 36 hours after administration of sofosbuvir and ribavirin using a high-performance liquid chromatography-mass spectrometry bioanalytical technique (QPS LLC).
IL28B Genotyping
Genotyping of the IL28B single-nucleotide polymorphism rs12979860 has been previously shown to be associated with treatment outcome.16- 17Whole blood was collected using PAXgene Blood DNA tubes (Qiagen) and stored at -80°C until DNA extraction. DNA was extracted using the Paxgene Blood DNA Kit (PreAnalytiX, a Qiagen/BD Company). The IL28B genotype was conducted in a blinded fashion on DNA specimens using the 5' nuclease assay with IL28B-allele-specific TaqMan probes (ABI TaqMan allelic discrimination kit) and the ABI7500 Real-Time PCR system (Applied Biosystems). The IL28B genotyping was classified as either favorable (CC genotype) or unfavorable (CT or TT genotypes).18
454 Deep Sequencing for the Detection of S282T NS5B Mutation
The major mutation shown to confer resistance to NS5B drugs including sofosbuvir is an S282T mutation.19 The HCV viral RNA was extracted from baseline plasma using QIAamp Viral RNA Mini (Qiagen) followed by reverse transcription-polymerase chain reaction to amplify complementary DNA. Using the Genome Sequencer FLX system, 454 Deep sequencing was then performed in the NS5B region to determine the presence of putative resistance-associated variants to sofosbuvir, including S282T.
Liver Biopsy
All participants had undergone a liver biopsy within 3 years of enrollment, and an optional research biopsy was offered after treatment completion (within 2 weeks of drug cessation). Histopathological assessments were performed by a single pathologist in a nonblinded fashion at the time of biopsy and staged according to the Knodell-HAI scoring system.20
Clinical End Points
The primary study end point was the proportion of participants with undetectable HCV viral load 24 weeks after treatment completion (SVR24). Secondary efficacy end points included the proportion of participants with undetectable HCV viral load at specified time points during and after treatment. Safety end points included frequency and severity of adverse events, discontinuations due to adverse events, and safety laboratory changes.
Modeling Viral Kinetics, Pharmacokinetics, and Pharmacodynamics
Pharmacokinetics, pharmacodynamics, and viral kinetic modeling of sofosbuvir in the 20 randomized participants in the substudy were calculated using previously described techniques.21- 23 An estimation of mean and maximum drug efficacy, infected cell loss rate, and loss rate of free virus was generated with this model.
Statistical Analysis
Although the primary interest was the per-protocol analysis, we also present the intention-to-treat analysis of all randomized participants because these results are more readily generalizable. The per-protocol analysis included all participants who received at least 8 weeks of the study drug. For efficacy analysis, missing data points were deemed a success if the immediately preceding and subsequent time points were successful; otherwise, data points were termed as failures. Participants who had missing data due to premature discontinuations were considered failures from the point of discontinuation. Comparisons were analyzed using the nonparametric Wilcoxon rank sum test for continuous outcomes and Fisher exact test for binary outcomes. A bivariable logistic regression model of baseline characteristics was used to identify factors associated with relapse. All P values were 2-tailed and were considered significant only when lower than .05. Analysis was performed using PRIZM 8.0 (GraphPad Software), SAS (SAS Institute Inc), STAT-CRUNCH, and S-Plus 8.0 (Statistical Sciences Inc). Sample size was calculated using an assumed early response rate of 90% for the weight-based group vs 85% for the low-dose group. With 50 participants, the study would be able to estimate the difference in early virologic response proportions to within plus or minus 0.18. There was a substantial gain in precision (from an accuracy of ± 0.24 to ± 0.18) from increasing the sample size from 15 to 25 per group but diminishing returns after a sample size of 25.
RESULTS
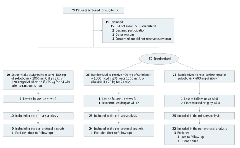
Seventy-nine participants were screened and 60 were enrolled in this study (10 participants in part 1 [proof of concept] and 50 participants in part 2 [randomized portion]; Figure). All results including treatment response and safety in the 10 nonrandomized participants are shown in eTable 1 and eTable 2 in the Supplement.
Figure.
Study Flow Diagram
The first 10 were sequentially enrolled from eligible participants in an open-label exploratory group. Relapse is determined at any time after end of treatment response but brior to sustained virologic response at 24 weeks.The patient who discontinued at week 12 was still included in both analyses as having reached sustained virologic response at 24 weeks.
(Click table to enlarge)
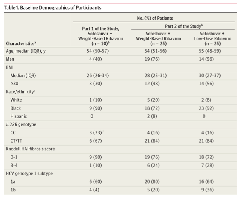
Baseline Characteristics of Participants
Baseline characteristics were similar among treatment groups (Table 1). Eighty-three percent of the participants were black; 66%, men; and 48%, body mass index greater than 30 (calculated as weight in kilograms divided by height in meters squared); 81% had the IL28 CT or TT genotype; 70%, GT-1a genotype; 23%, advanced liver disease; and 62%, baseline HCV RNA levels greater than 800 000.
(Click figure to enlarge)
Virologic Response
Twenty-four participants (96%) in each group achieved viral suppression by week 4. Four participants discontinued the study drug by week 8 due to nonadherence (1 in the weight-based group; 3 in the low-dose; Figure). One patient declined to continue study drug past week 12, but his viral load remained undetectable 24 weeks after stopping treatment. He is included in the final analysis. A total of 7 participants (28%) in the weight-based group and 10 (40%) in the low-dose group relapsed after treatment completion leading to SVR24 rates of 68% (95% CI, 46%-85%) in the weight-based group and 48% (95% CI, 28%-69%; P = .20) in the low-dose group (Table 2). A within-cohort comparison of baseline factors related to SVR was performed and is shown in eTable 3 in the Supplement. Deep sequencing of all baseline samples showed no S282T resistant mutant.
Viral Kinetic, Pharmacokinetic, and Pharmacodynamic Modeling
All participants experienced a rapid decline in plasma HCV RNA. A viral kinetic model over the first 50 days of treatment of all randomized participants showed no differences in viral decay based on ribavirin dose or baseline characteristics (eFigure 1 and eFigure 2 in the Supplement). However, a fully fitted pharmacokinetics-viral kinetics model of a subset of 20 participants (10 in the low-dose group and 10 in the weight-based group) showed a significantly slower loss rate of free virus (clearance) in relapsers than participants who achieved SVR (clearance, 3.57 vs 5.60 per day; P = .009). There were no observable differences in viral decay, drug efficiency, loss rate of infected cells, or loss rate of infectious virus based on baseline characteristics (eTable 4 in the Supplement).
Histologic Response
Twenty-nine participants (58%) had paired liver biopsies with an improvement in inflammation in 27 participants (93%) with a median drop of 5 points (15-point scale; eFigure 3A and B in the Supplement). In parallel with HCV RNA decline, there was rapid improvement of alanine aminotransferase levels with 77% normalizing by day 7 and 98% by day 14. A similar pattern was observed with aspartate aminotransferase levels (eFigure 4A in the Supplement).
Safety
The combination of sofosbuvir and ribavirin was safe and well tolerated with no death or discontinuation of treatment due to adverse events. The most frequent adverse events were headache, anemia, fatigue, and nausea, the severity of which ranged from mild to moderate (Table 3). There were 7 grade 3 events.
Participants in the weight-based group experienced a higher incidence of hemoglobin decline, which was maintained through week 12, than did participants in the low-dose group (week 4, 37% vs 4%; P = .005; week 12, 39% vs 4%; P = .01; eFigure 4B, in the Supplement). Eight participants (5 in the weight-based group) underwent ribavirin dose reduction for decreased hemoglobin including 3 with a history of coronary artery disease with ribavirin reduction instituted at a hemoglobin level of less than 12 g/dL. There was no use of erythropoietin-stimulating agents in this study. No major biopsy-related complications were observed.
Characteristics Associated With Relapse
Bivariable analysis of baseline factors showed that in all randomized participants who completed treatment, the odds of relapse was significantly higher in participants who were male (odds ratio [OR], 6.09; 95% CI, 1.17-31.6), had advanced fibrosis (OR, 4.27; 95% CI, 1.10-16.54), and baseline HCV RNA greater than 800 000 IU/mL (OR, 5.74; 95% CI, 1.35-24.38; Table 4). Given the small number of events and the exploratory nature of the stepwise analysis that determined variables used in the model, only the bivariable model results are reported herein.

(Click table to enlarge)
DISCUSSION
Although the treatment of HCV is rapidly evolving, several questions remain unanswered. This study demonstrates the efficacy of an interferon-free regimen in a traditionally difficult-to-treat population while exploring the reasons for treatment relapse. In this study, treatment of chronic HCV infection with a single directly acting antiviral agent (sofosbuvir) and weight-based ribavirin resulted in a high SVR rate in a population with unfavorable traditional predictors of treatment response compared with reported rates with currently used interferon-based therapy in similar populations.7- 8Compared with previous trials testing boceprevir and telaprevir, this population had a higher prevalence of unfavorable traditional predictors of treatment response including black race (80% vs 7%-15%); genotype 1a (70% vs 60%-64%); advanced fibrosis (24% vs 9%-20%); and body mass index greater than 30 (49% vs 22%).7- 8 The overall SVR rate achieved by participants who received sofosbuvir in combination with weight-based ribavirin in our study was 68% compared with the 84% reported in a recent New Zealand study of sofosbuvir and weight-based ribavirin in a predominantly white, treatment-naive population.11 Because treatment of HCV is evolving from an interferon-based combination therapy to an all-oral, interferon-free directly acting antiviral agent regimen, these results are encouraging and provide important information regarding the expected treatment responses in a population representative of the US epidemic.
In an exploratory bivariable model, we found that the baseline factors of male sex, advanced liver disease, and high baseline HCV RNA were associated with relapse. The association of advanced liver disease with higher odds of relapse is similar to that described with direct-acting antiviral agent interferon-based regimens.6- 8 In this regard, 7 of the 13 participants (54%) with advanced liver fibrosis treated in this study relapsed including all 4 participants with cirrhosis. Future studies are warranted to evaluate the efficacy of sofosbuvir and ribavirin regimens in participants with advanced fibrosis.
There were no cases of viral breakthrough while receiving therapy in participants treated with sofosbuvir and ribavirin similar to what has been reported in prior studies.11 Comprehensive analysis of baseline plasma HCV quasi-species by 454 deep sequencing failed to detect the characteristic S282T mutants previously associated with resistance to sofosbuvir.
The kinetics of HCV decline during interferon and ribavirin therapy has been previously described as a predictor of SVR.21- 22 Because interferon-free directly acting antiviral agent therapy is entirely based on achieving maximum suppression of HCV replication, we sought to explore the effect of early HCV viral kinetics, pharmacokinetics, and pharmacodynamics on therapeutic response. Although there were no significant differences in viral kinetics or pharmacokinetics between the weight-based ribavirin and low-dose ribavirin groups, the viral kinetics-pharmacodynamics model demonstrated a significantly slower loss rate of infectious virus in participants who subsequently relapsed. The mechanism of viral relapse in these participants remains elusive and future research will be focused on identifying the biological basis for incomplete clearance of HCV in these participants.
Limitations of this study include the relatively small sample size in each group and a higher, though small, increase in the number of discontinuations with low-dose ribavirin. Due to the small size, associations described are preliminary in nature and require further evaluation in larger studies.
Ribavirin is associated with significant adverse events13,23 but appears to be essential for optimal response to interferon-based and certain directly acting antiviral agent therapies.11,14- 15 Although our study did not show a significant association between treatment response and ribavirin dosing, it remains important to determine the optimal dose and role of ribavirin in the treatment of chronic HCV infection in larger interferon-free studies. In conclusion, treatment with a 24-week regimen of sofosbuvir and ribavirin resulted in an SVR rate of 68% in the weight-based ribavirin regimen and 48% in the low-dose ribavirin regimen among patients with chronic HCV and unfavorable traditional predictors of treatment response who are representative of the demographics of the US HCV epidemic. The delineation of the host and viral factors associated with treatment relapse with different directly acting antiviral agent interferon-free regimens needs to be further assessed. As new direct-acting antivial agent regimens are being evaluated, it is important that these studies involve populations most affected by the disease.
Source