ACS Medical Chemistry Letters
Ann D. Kwong *
InnovaTID, Inc., 125 Cambridge Park Drive, Cambridge,
Massachusetts 02140, United States
ACS Med. Chem. Lett., 2014, 5 (3), pp 214–220
DOI: 10.1021/ml500070q
Publication Date (Web): February 27, 2014
Copyright © 2014 American Chemical Society
*E-mail: ann.kwong@innovatid.org. Phone: 617-501-3453.
Abstract
The progress in HCV therapy in the last three years is similar to the progress that took HIV therapy  14 years. We are at the brink of approval for an all-oral drug combination that is dosed once daily as a single pill, has >95% efficacy, and is well tolerated. This article summarizes the path to this success and the challenges still ahead.
14 years. We are at the brink of approval for an all-oral drug combination that is dosed once daily as a single pill, has >95% efficacy, and is well tolerated. This article summarizes the path to this success and the challenges still ahead.
Looking back at the last 20 years in HCV drug discovery, I am struck by how fast the progress has been in recent years, and how slow it was in the early years. This viewpoint will attempt to identify some of the factors that contributed to both effects, in the hope that the knowledge will inform and accelerate future drug discovery.
HCV Revolution
The past decade was an exciting time in the world of hepatitis C virus (HCV) drug discovery. By 2011, over 50 companies worked on HCV, and in 2013, more than a dozen were conducting phase 2 and phase 3 clinical trials.(1) The past few years saw rapid progression toward “the Holy Grail” of HCV: an all-oral, highly tolerable therapy with a >95% cure rate for all chronic HCV infections. The FDA approved two first-generation HCV protease inhibitors in 2011, then a second-generation HCV protease inhibitor and a HCV polymerase nucleotide inhibitor in 2013. In addition, excellent phase 3 data has been reported for several all-oral drug combinations, which may launch in 2014 (Figure 1). Treatment success rates improved from  40% in 2001 to
40% in 2001 to  79% in 2011 and
79% in 2011 and  89% in 2013, with multiple phase 3 trials with oral combinations reporting efficacy rates above 95%. Remarkably, increased efficacy of the new regimens came without increased toxicity or less tolerability.
89% in 2013, with multiple phase 3 trials with oral combinations reporting efficacy rates above 95%. Remarkably, increased efficacy of the new regimens came without increased toxicity or less tolerability.

Figure 1. Nonhead-to-head comparison of efficacy vs duration of HCV treatment regimens for different HCV genotypes as a function of the year of regulatory approval. The data reported are derived from multiple sources and different clinical trials and are shown side-by-side for pedagogical purposes and should not be construed to be definitive numbers. Efficacy (blue columns): the % sustained viral response (SVR), i.e., the % cure rate. A patient is said the be “cured” or have a “SVR” if their plasma HCV RNA levels become undetectable during treatment and remain undetectable for 24 weeks after the end of treatment. Duration (yellow columns): the total length of time a patient is on antiviral therapy, not just the length of time a patient is receiving a direct acting antiviral (DAA) drug. All the treatment durations shown in the graph were 12, 24, or 48 weeks. Geno, genotype; IFN, interferon; PR, pegylated interferon alfa 2a/b and ribavirin; RBV, ribavirin; DAA, direct acting antiviral.
Unmet Medical Need
HCV is a blood borne pathogen that chronically infects  170 million people. Thorough screening of blood supplies has significantly reduced new infections. Globally, the incidence of disease and the market for HCV drugs are inversely related. The Western Pacific, Southeast Asia, and Africa regions have the highest prevalence, with
170 million people. Thorough screening of blood supplies has significantly reduced new infections. Globally, the incidence of disease and the market for HCV drugs are inversely related. The Western Pacific, Southeast Asia, and Africa regions have the highest prevalence, with  126 million infections and no access to the new HCV drugs. However, the US and EU have
126 million infections and no access to the new HCV drugs. However, the US and EU have  13 million chronic HCV infections, which are the target market for the new HCV drugs. Worldwide, treatment is complicated because HCV occurs in 6 major genotypes, which affect drug sensitivity and are unevenly distributed geographically and between income levels (Figure 2B).
13 million chronic HCV infections, which are the target market for the new HCV drugs. Worldwide, treatment is complicated because HCV occurs in 6 major genotypes, which affect drug sensitivity and are unevenly distributed geographically and between income levels (Figure 2B).
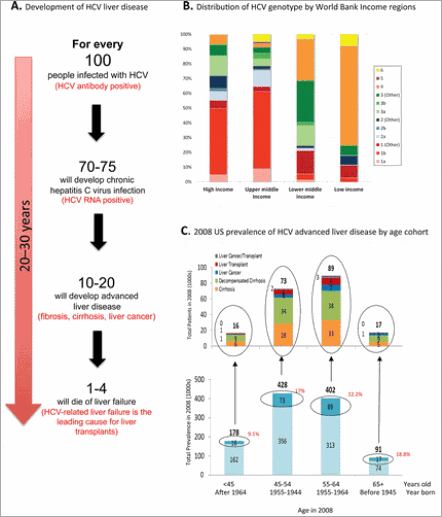
Figure 2. Breakdown of HCV-associated advanced liver disease in the US in 2008. (A) Time line of the development of HCV-associated liver disease. (B) Distribution of HCV genotypes by World Bank income regions. HCV has evolved into 6 major strains or genotypes that differ genetically from each other, which may result in a slightly different amino acid sequence in the region comprising and supporting the binding site of an antirviral drug. Therefore, drugs developed to inhibit genotype 1 HCV, the major genotype found in high-income and upper-middle income regions, might not inhibit genotypes found in the rest of the world such as genotypes 2–6. Panel B is taken from a poster entitled “Global distribution of HCV by prevalence and genotype” distributed by the Center for Disease Analysis (www.centerforda.com) at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Nov 1–5, 2013, Washington, DC (reproduced with permission from Homie Razavi). (C) 2008 US prevalence of HCV advanced advanced liver disease by age cohort. Upper graph: Each column breaks out the number of people with different types of advanced liver disease by age cohort (i.e., the blue box in the lower graph). The order of the types of advanced liver disease is the same for each age cohort. The number of patients (in thousands) with cirrhosis is shown in orange, decompensated cirrhosis is shown in green, liver cancer is shown in dark blue, liver transplant is shown in red, and liver cancer/transplant is shown in purple. Lower graph: Each column denotes the total number (in thousands) of people chronically infected with HCV in the US in 2008 as a function of age cohort. The upper portion of each column (dark blue) denotes the number of people with advanced liver disease.
A person can have a chronic HCV infection for decades with no obvious symptoms. Unfortunately, the longer a person is infected with HCV, the higher the chance of developing liver fibrosis, cirrhosis, failure, portal hypertension, and cancer. Typically, these symptoms occur over a period of 20–30 years (Figure 2A).
The good news is that, unlike infections with HIV and HBV, HCV can be cured. The bad news is that the majority of people infected do not know they have an HCV infection. In 2008, of the 2.68 million people in the US with chronic HCV infection, 59% were undiagnosed.(2) Figure 2C (bottom) shows the prevalence of advanced liver disease (dark blue box) in the diagnosed population as a function of age. These data reveal that baby boomers, born between 1945 and 1964, account for 75% of newly diagnosed chronic HCV patients and 84% of advanced liver disease patients. The upper graph in Figure 2B shows that the majority of advanced liver disease is decompensated cirrhosis, followed by cirrhosis. For perspective, although liver cancer represents a small fraction of advanced liver disease cases, it represents the fastest growing cancer death rate in the US. These data suggest that baby boomers are at high risk of progressing to advanced liver disease and support the CDC’s recommendation for targeted screening of this cohort.
Three Waves of HCV Drug Creation
Over the last 27 years, advances in three areas have enabled direct-acting antiviral drugs for HCV to move forward: (i) research and discovery, (ii) development, and (iii) approval and commercialization (Figure 3).
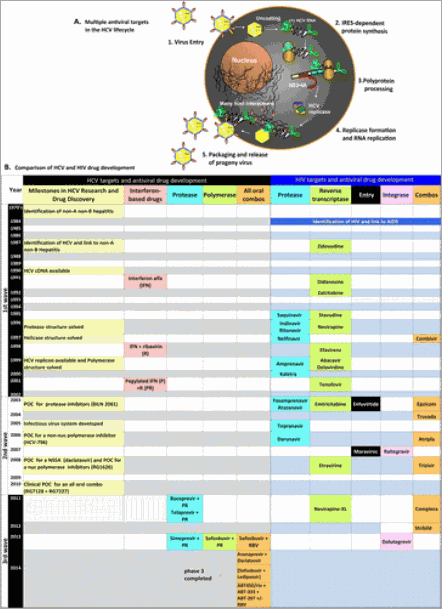
Figure 3. HCV antiviral targets and a comparison of HCV and HIV drug creation timelines. (A) Schematic illustration of multiple steps in the HCV virus lifecycle that can be the target of an antiviral drug. IRES, internal ribosome entry site. (B) Comparison of HIV and HCV drug development time lines. POC, proof of concept; non-nuc, non-nucleotide; nuc, nucleotide; IFN, interferon alfa 2a/b; R or RBV, ribavirin; P, pegylated interferon alfa 2a/b; PR, pegylated interferon alfa 2a/b plus ribavirin.
First Wave (Research and Discovery) of HCV Drug Creation
The first wave from 1987–2002 set the foundation for the clinical development work that followed (Figure 2B). HCV replication can be inhibited at several different points in the lifecycle, by targeting either viral or host functions. As a consequence, more than 40 different treatment options are either in the market or in clinic trials; reviewed in refs 1 and 3. The crystal structures of the HCV NS3·4A protease and the NS5B RNA polymerase set a strong foundation for rational structure-based drug design, which in turn was critical for optimizing protease potency and allosteric binding. The development of the HCV replicon system in 1999(4) fueled an explosion in the scientific understanding of the HCV life cycle and enabled the development of high-throughput replicon cell-based assays. The replicon assay was used to identify a potent novel class of inhibitors that target the NS5A replication complex.(5)
Comparison of HCV and HIV Drug Development
In the past few years, rapid advances in the development of HCV drugs have looked like HIV drug development on speed, but is this a fair comparison? As shown in Figure 3, the first HIV drug was approved 3 years after the virus was identified as the etiological agent of AIDS. Since that time, the FDA approved 26 different direct-acting antiviral drugs or combinations targeting four different HIV drug targets (protease, reverse transcriptase, entry, and integrase).(1, 6) In contrast, it took 24 years after HCV was discovered for the first direct-acting antiviral drugs to be approved and 27 years for four combinations to be approved against two HCV targets (protease and polymerase).
Why Did It Take so Long for HCV DAAs to Be Developed?
Multiple factors that slowed the development of direct-acting HCV drugs included:4
• low perceived market value and a poor potential return on investment
• no tools to test the ability of compounds to inhibit HCV replication in vitro in cultured cells
• expensive licensing fees for reagents
• low pressure from patient lobbies
• a belief that all-oral treatment for HCV would never work because HCV was a liver disease, not a viral disease, and interferon would always be required.
• a poor understanding of the nature of HCV resistance to drugs led to the misconception that HCV could be successfully treated with monotherapy drugs like HSV or CMV drugs. In contrast, HCV is more like HIV and requires a multidrug combination to suppress resistant variants.
• a belief that all nucleoside/tide polymerase inhibitors are toxic, despite the fact that they are the backbone of HIV, HSV, CMV, and HBV antiviral therapy.
What Factors Increased the Speed of Research and Discovery for HCV Direct-Acting Antiviral Drugs?
The mantra of those who moved these drug combinations forward the fastest was “It’s the virus, stupid,” focusing on inhibiting HCV at multiple points in the virus lifecycle (Figure 3A) without going through a combination phase with PEGylated interferon and ribavirin. Finally, a critical, but less well-known, decision by regulatory authorities to permit drug companies to use the HCV replicon assay instead of an infectious virus assay or an animal model significantly increased the speed of development of all-oral combos.
Second Wave (Development) of HCV Drug Creation
The second wave lasted between 2003–2010 when HCV clinical virology and the clinical development path for HCV direct-acting antiviral drugs were set. The early development of the interferon-based drugs demonstrated that HCV could be cured and set the paradigm for treating a patient for a period of time after their HCV virus becomes undetectable and then monitoring for viral relapse after the end of treatment.
What Factors Increased the Speed of Developing HCV Direct-Acting Antiviral Drug Development?
With the dual goal of increasing the cure rate and reducing the duration of treatment, researchers designed novel clinical trials to explore the two parameters in the same study, rather than sequentially as had been traditional. In addition, clinical pharmacology modeling based on viral kinetics, population analyses, and pharmacokinetics was used to justify study design and to add subpopulations to the product label that were not explicitly tested. HIV research led to the use of HCV viral RNA levels as a primary end point in clinical trials, instead of following an indirect effect (ALT levels), and to the use of combinations of direct-acting antiviral drugs to combat resistance. This is exemplified by phase 3 data that revealed that three different combinations could cure patients with HCV: a protease inhibitor plus a NS5A inhibitor; a polymerase nucleotide inhibitor plus a NS5A inhibitor; and a protease inhibitor plus a polymerase allosteric inhibitor plus a NS5A inhibitor (Figure 1 and Table 1).
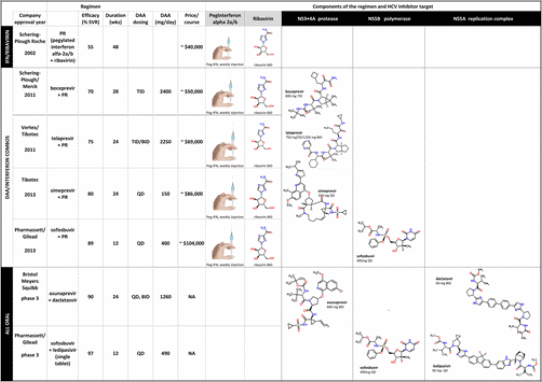
Table a Three classes of HCV regimens (pegylated interferon + ribavirin (PR)), DAA + PR, and all oral DAA combos) are compared on the basis of efficacy, duration, dosing interval, dose size, price, mechanism of action (target), and compound structure. Only genotype 1 results and regimens with publically available compound structures were included. The price listed is a best-guess estimate based on publically available sources. SVR, sustained viral response; wks, weeks; DAA, direct acting antiviral; TID, 3 times a day dosing; BID, twice daily dosing; QD, once daily dosing; mg, milligram; NA, not available.
Another important factor that accelerated the development of HCV clinical study design and resistance studies was the founding of HCV DrAG, a collaboration between the major stake holders: academia, pharmaceutical companies, regulators, and community representatives.(7) HCV DrAG played a major role in facilitating the creation of new FDA and EMA guidances, which permit the combination of two or more unapproved drugs.
Short monotherapy proof-of-concept studies performed with inhibitors of HCV protease, NS5A, and HCV polymerase demonstrated that HCV replication could be inhibited in the absence of PEGylated interferon and ribavirin. Figure 3B lists some of the groundbreaking proof of concept studies. None of the development programs that were the first to demonstrate clinical proof of concept actually became approved drugs, illustrating the difficulty and risk inherent in creating drugs.
The first three HCV direct-acting antiviral drugs to be approved, telaprevir, boceprevir, and simeprevir, were protease inhibitors that had a low barrier to resistance, requiring them to be combined with PEGylated interferon and ribavirin to treat genotype 1 patients (Table 1). A nucleotide inhibitor of HCV polymerase, sofosbuvir followed, also in combination with PEGylated interferon and ribavirin for genotypes 1 and 4 patients. Sofosbuvir has a high barrier to resistance and was approved as an all-oral combination with ribavirin for patients with genotypes 2 and 3. The next two classes of compounds were polymerase non-nucleotide allosteric inhibitors (ABT-333) and NS5A replication inhibitors (daclatasvir, lepidipasvir, and ABT-267). In order to increase the barrier to resistance, the all-oral combinations shown in Figures 1 and 3 and Table 1 all include direct-acting antiviral agents with at least two different mechanisms of action. By focusing directly on developing an all-oral approach, bypassing developing a combination with PEGylated interferon and ribavirin, the time required to launch an all-oral combination was shortened.
Third Wave (Approval and Commercialization) of HCV Drug Creation
The third wave started in 2011. In this wave the FDA approved the first new antiviral regimen for chronic HCV in a dozen years. Two first generation direct-acting antiviral HCV protease inhibitors, telaprevir and boceprevir, were approved for used in combination with PEGylated interferon and ribavirin for patients with genotype 1 chronic HCV infection.(8) Patients and prescribers welcomed the greater efficacy and shorter treatment compared to PEGylated interferon and ribavirin (Figure 1 and Table 1).
It is well-known that the long path to drug approval has a high attrition rate. Only 10–20% of all compounds that enter the clinic are approved. Less well-known is the fact that  80% of approved drugs are not profitable.(9) What drives market success? Compared to boceprevir, telaprevir had similar potency, slightly better efficacy, slightly worse side effects, a simpler dosing regimen, lower pill burden, a higher price, and a newly built commercial operation. No one predicted what happened: telaprevir significantly outsold boceprevir with $2.11 billion for telaprevir vs $0.114 billion for boceprevir for the period between Q3 2011 to Q4 2012 and become the fastest drug in history to reach a billion dollars in sales. Why was telaprevir so successful? In my opinion, multiple reasons include, but are not limited to, higher efficacy, faster decline in HCV RNA in the first four weeks, lower pill burden, better dosing regimen, simpler treatment paradigm, experienced sales force and account managers, good patient copay support, and a great scientific story.
80% of approved drugs are not profitable.(9) What drives market success? Compared to boceprevir, telaprevir had similar potency, slightly better efficacy, slightly worse side effects, a simpler dosing regimen, lower pill burden, a higher price, and a newly built commercial operation. No one predicted what happened: telaprevir significantly outsold boceprevir with $2.11 billion for telaprevir vs $0.114 billion for boceprevir for the period between Q3 2011 to Q4 2012 and become the fastest drug in history to reach a billion dollars in sales. Why was telaprevir so successful? In my opinion, multiple reasons include, but are not limited to, higher efficacy, faster decline in HCV RNA in the first four weeks, lower pill burden, better dosing regimen, simpler treatment paradigm, experienced sales force and account managers, good patient copay support, and a great scientific story.
As is common with first generation drugs, there was significant room for improvement. Drawbacks of telaprevir and boceprevir included low efficacy rates in some patient populations, high pill burdens, significant serious side effects, and three times a day dosing (Table 1). The beginning of the endgame in the US and EU was revealed when Gilead announced phase 3 results, in which genotype 1 chronic HCV patients treated with 12-weeks of a once-daily fixed dose combination of an HCV polymerase nucleoside inhibitor (sofosbuvir) and NS5A inhibitor (ledipasvir) achieved a 96% cure rate.
Next Hurdle(s)
Now that potent and safe HCV direct-acting antiviral drugs are in hand, what are some of the outstanding issues?
Diagnosis and Treatment
In the US, most people with chronic HCV infection do not know they are infected. If these patients are not treated, many will develop advanced liver disease that will be costly and may be impossible to cure. An analysis of the HCV-associated nonpharmacological costs in the US between 2007 and 2009 showed that the rise in advanced liver disease more than doubled the overall growth in US healthcare costs (9.4% vs 4.3%).(2) However, if all of the currently undiagnosed people were to seek treatment, the current medical infrastructure will be overwhelmed. Now is the time to invest in diagnosis and treatment to avert a future avalanche of HCV-related advanced liver disease and associated costs.
Cost
The all-oral HCV therapy, highly efficacious and tolerable in the majority of patients, will become available in 2014. Like all new drugs, the new HCV treatment regimens are expensive. In the developed world, finding a price that works for all parties, patients, providers, payers, governments, and the pharmaceutical company, will be critical. The majority of people with chronic HCV infection do not live in the US, EU, and Japan, the primary market for current HCV drugs. We need a way to give patients in resource-poor countries access to effective HCV therapy despite their inability to pay high prices. I hope our industry will rise to the challenge to apply similar efforts to find a second Holy Grail: affordable access to curative HCV therapy for all, regardless of their country of residence or economic status.
Views expressed in this editorial are those of the author and not necessarily the views of the ACS.
The authors declare no competing financial interest.
Acknowledgment
Many thanks to Amy Juodawlkis, Rosemary Camilleri, Alan Collis, and Daša Lipovšek for editorial assistance.
Abbreviations
AIDS acquired immunodeficiency syndrome
ALT alanine liver transaminase
CMV cytomegalovirus
EMA European Medicines Agency
EU European Union
FDA US Food and Drug Administration
HBV hepatitis B virus
HCV hepatitis C virus
HCV DrAG HCV drug development group
HIV human immunodeficiency virus
NS nonstructural
RNA ribonucleic acid
This article references 9 other publications.
References
1. Clayden, P., Collins, S., Daniels, C., Frick, M., Harrington, M., Horn, T., Jefferys, R., Kaplan, K., Lessem, E., and Swan, T., 2013 Pipeline Report. In HIV, Hepatitis C (HCV), and Tuberculosis (TB) Drugs, Diagnostics, Vaccines, Preventive Technologies, Research toward a Cure, and Immune-based and GeneTherapies in Development; Benzacar, A., Ed.; HIV i-Base and Treatment Action Group: New York, 2013; p 295. http://www.pipelinereport.org (accessed January 29, 2014).
2. Zalesak, M.; Francis, K.; Gedeon, A.; Gillis, J.; Hvidsten, K.; Kidder, P.; Li, H.; Martyn, D.; Orne, L.; Smith, A.; Kwong, A.Current and future disease progression of the chronic HCV population in the United States PloS One 2013, 8 ( 5) e63959 [CrossRef], [PubMed]
3. Hunt, D.; Pockros, P.What are the promising new therapies in the field of chronic hepatitis C after the first-generation direct-acting antivirals? Curr. Gastroenterol. Rep. 2013, 15 ( 1) 303 [CrossRef], [PubMed], [CAS]
4. Lohmann, V.; Korner, F.; Koch, J.; Herian, U.; Theilmann, L.; Bartenschlager, R.Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line Science 1999, 285 ( 5424) 110– 3[CrossRef], [PubMed], [CAS]
5. Gao, M.; Nettles, R. E.; Belema, M.; Snyder, L. B.; Nguyen, V. N.; Fridell, R. A.; Serrano-Wu, M. H.; Langley, D. R.; Sun, J. H.; O’Boyle, D. R., II; Lemm, J. A.; Wang, C.; Knipe, J. O.; Chien, C.; Colonno, R. J.; Grasela, D. M.; Meanwell, N. A.; Hamann, L. G.Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect Nature 2010, 465 ( 7294) 96– 100[CrossRef], [PubMed], [CAS]
6. Flexner, C.HIV drug development: the next 25 years Nat. Rev. Drug Discovery 2007, 6 ( 12) 959– 66 [CrossRef], [PubMed], [CAS]
7. Forum for Collaborative HIV Research. HCV Drug DevelopmentAdvisory Group. http://www.hivforum.org/projects/hcv-drug-development-advisory-group.
8. Jacobson, I. M.; Pawlotsky, J. M.; Afdhal, N. H.; Dusheiko, G. M.; Forns, X.; Jensen, D. M.; Poordad, F.; Schulz, J.A practical guide for the use of boceprevir and telaprevir for the treatment of hepatitis C J. Viral Hepatitis 2012, 19 ( Suppl 2) 1– 26 [CrossRef], [PubMed], [CAS]
9. Dorato, M. A.; Buckley, L. A. Toxicology Testing in Drug Discovery and Development. In Current Protocols in Toxicology; Wiley: New York, 2007; Chapter 19, Unit 19.1.
Source

 14 years. We are at the brink of approval for an all-oral drug combination that is dosed once daily as a single pill, has >95% efficacy, and is well tolerated. This article summarizes the path to this success and the challenges still ahead.
14 years. We are at the brink of approval for an all-oral drug combination that is dosed once daily as a single pill, has >95% efficacy, and is well tolerated. This article summarizes the path to this success and the challenges still ahead.